09 Nov 09:03
by Pedro Alonso, Pilar Pardo, Alicia Galván, Francisco J. Fañanás, Félix Rodríguez
Abstract
Cyclic alkenyl triflates are useful intermediates in organic synthesis usually synthesized from ketones through a reaction involving enolization and trapping with a triflating agent. This sequence suffers from some stereochemical drawbacks owing to the basic conditions required. Herein, we describe a new acid-mediated cationic cyclization reaction of enyne derivatives (or alkynols) to access cyclic alkenyl triflates. This new atom-economical process is high yielding, scalable, technically very simple, proceeds without the need of any metallic reagent or catalyst, and more importantly, it complements and challenges conventional methodologies. We have also developed new biomimetic cationic cyclization reactions to yield interesting polycyclic compounds. As a demonstration of the potential of this method in the context of total synthesis, we have synthesized two terpenes: austrodoral and pallescensin A. Using the cationic cyclization in the key step of the synthetic routes allowed the synthesis of these natural products in a very simple, concise, scalable, and efficient way.

Cyclic alkenyl triflates are easily available through a new reaction based on a cationic cyclization process. Extension of the method to biomimetic polycyclization reactions allows the selective synthesis of interesting polycyclic core skeletons, including the terpenes austrodoral and pallescensin A.
09 Sep 08:59
by Manuel Basauri-Molina, Dide G. A. Verhoeven, Arnoldus J. van Schaik, Henk Kleijn, Robertus J. M. Klein Gebbink
Abstract
A series of Grubbs-type catalysts that contain lipase-inhibiting phosphoester functionalities have been synthesized and reacted with the lipase cutinase, which leads to artificial metalloenzymes for olefin metathesis. The resulting hybrids comprise the organometallic fragment that is covalently bound to the active amino acid residue of the enzyme host in an orthogonal orientation. Differences in reactivity as well as accessibility of the active site by the functionalized inhibitor became evident through variation of the anchoring motif and substituents on the N-heterocyclic carbene ligand. Such observations led to the design of a hybrid that is active in the ring-closing metathesis and the cross-metathesis of N,N-diallyl-p-toluenesulfonamide and allylbenzene, respectively, the latter being the first example of its kind in the field of artificial metalloenzymes.

Artificial metalloenzymes for olefin metathesis have been created by the covalent anchoring of a Grubbs catalyst in the active site of the enzyme host (cutinase). Owing to the strategic positioning of the catalyst, strong protein scaffold–catalyst interactions occur, which affect the steric hindrance in the hybridization or the catalytic activity for different catalyst structures. Activity was found in the ring-closing metathesis of N,N-diallyl p-toluenesulfonamide and in the formal cross-metathesis of allylbenzene, which is the first reported example with artificial enzymes.
20 Jan 09:29
Chem. Commun., 2015, 51,3407-3410
DOI: 10.1039/C4CC09590A, Communication
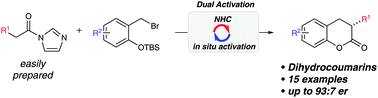
Anna Lee, Karl A. Scheidt
A highly efficient asymmetric formal [4+2] annulation for the synthesis of dihydrocoumarins has been developed via an in situ activated NHC catalysis.
The content of this RSS Feed (c) The Royal Society of Chemistry

14 Jan 11:02
by Youngeun Jung and Ikyon Kim

The Journal of Organic Chemistry
DOI: 10.1021/jo502745j

13 Jan 09:21
Chem. Commun., 2015, 51,2731-2733
DOI: 10.1039/C4CC09003A, Communication
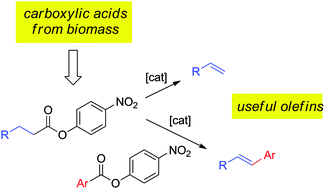
Alex John, Levi T. Hogan, Marc A. Hillmyer, William B. Tolman
Catalytic method employs "masked" carboxylic acids to yield alkenes, via decarbonylation and/or C-C coupling of activated esters.
The content of this RSS Feed (c) The Royal Society of Chemistry

30 Sep 10:34
Chem. Commun., 2014, 50,14364-14366
DOI: 10.1039/C4CC07376B, Communication
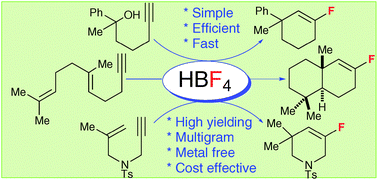
Pedro Alonso, Pilar Pardo, Francisco J. Fananas, Felix Rodriguez
Tetrafluoroboric acid promotes a fluorinative carbocyclization reaction to give cyclic alkenyl fluorides in a process where the incorporation of the fluorine atom occurs through the nucleophilic addition of a fluoride anion.
The content of this RSS Feed (c) The Royal Society of Chemistry

26 Sep 08:44
by Shoubhik Das, Felix D. Bobbink, Gabor Laurenczy, Paul J. Dyson
Abstract
N-methylation of amines is an important step in the synthesis of many pharmaceuticals and has been widely applied in the preparation of other key intermediates and chemicals. Therefore, the development of efficient methylation methods has attracted considerable attention. In this respect, carbon dioxide is an attractive C1 building block because it is an abundant, renewable, and nontoxic carbon source. Consequently, we developed a highly chemoselective, metal-free catalytic system that operates under ambient conditions for the N-methylation of amines.

The methylation of amines with CO2 as C1 source and Ph2SiH2 as reducing agent was achieved with an N-heterocyclic carbene (NHC) as the catalyst. The catalyst is tolerant toward a variety of functional groups (including esters and ethers, nitro, nitrile, and carbonyl groups, and unsaturated C![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) C bonds), uses commercially available reagents, and can be performed on a gram scale.
C bonds), uses commercially available reagents, and can be performed on a gram scale.
24 Sep 09:17
by Eva Tudela, Jairo González, Rubén Vicente, Javier Santamaría, Miguel A. Rodríguez, Alfredo Ballesteros
Abstract
An allylic gold(I) cation, proposed as key intermediate in the gold-promoted rearrangement of 1,5-enynes bearing a fixed conformation, has been detected and characterized by NMR spectroscopy. Moreover, its participation in the overall transformation was confirmed. Computational studies indicate that the gold-catalyzed transformation occurs through an uncommon rearrangement. Additionally, this study led us to isolate and characterize a stable homoantiaromatic carbocation.

No longer elusive: The allylic gold(I) cation 2, which has been proposed as an intermediate in the rearrangement of alkynylcyclopropanes (1) into alkynylcyclohexadienes (3), has been detected and characterized by NMR spectroscopy. Participation of 2 was supported experimentally and theoretically, and through these studies, a stable homo-antiaromatic carbocation was isolated and characterized.
28 Jul 07:31
by Olga Ekkert, Christopher B. Caputo, Conor Pranckevicius, Constantin G. Daniliuc, Gerald Kehr, Gerhard Erker, Douglas W. Stephan
24 Jul 16:34
by Nandini Sharma, Zhenghua Li, Upendra K. Sharma and Erik V. Van der Eycken

Organic Letters
DOI: 10.1021/ol5019079

24 Jul 09:43
by Jeffrey Santandrea, Anne-Catherine Bédard and Shawn K. Collins

Organic Letters
DOI: 10.1021/ol501898b

21 Jul 10:50
by Thomas C. Atack, Rachel M. Lecker and Silas P. Cook

Journal of the American Chemical Society
DOI: 10.1021/ja505199u

18 Jul 10:43
by Stephen E. Ammann, Grant T. Rice and M. Christina White

Journal of the American Chemical Society
DOI: 10.1021/ja503322e

15 Jul 14:11
by Mutsuyo Ohara, Yoshichika Hara, Tohru Ohnuki, Shuichi Nakamura
Abstract
An enantioselective three-component reaction of aldehydes, amines, and alkynes in water by using a bis(imidazoline)–CuI catalysts having a hydrophobic substituent and sodium dodecyl sulfate as a surfactant was developed. The reaction was applied to a broad range of aldehydes and alkynes to give optically active propargylamines with excellent yields (up to 99 %) and enantiomeric excesses (up to 99 % ee).

An enantioselective three-component reaction of aldehydes, amines, and aliphatic alkynes in water by using a copper(I) complex of 1,3-bis(imidazolin-2-ly)pyridine (pybim) to afford optically active propargylamines was developed (see scheme; DS=dodecylsulfate). The reaction was applied to a wide range of aldehydes and alkynes, giving products with excellent yields (up to 99 %) and enantiomeric excesses (up to 99 % ee).
14 Jul 10:42
by Abdessamad Grirrane, Eleuterio Álvarez, Hermenegildo García, Avelino Corma
Abstract
Care should be exercised when using CH2Cl2 as a solvent for reactions in which amines are a reagent, since undesirable deactivation of cationic copper(I) and gold(I) catalysts to form the corresponding inactive neutral chloride complexes [LMCl] (M=Cu or Au) can occur as a result of the generation of hydrogen chloride in the medium. CuI and AuI deactivation has been proved for the Mannich three-component coupling reaction. A series of CuI and AuI complexes with potential mechanistic implications were isolated and characterized by X-ray crystallography.

Proceed with caution! Care should be exercised when using CH2Cl2 as a solvent for reactions in which amines are a reagent, since undesirable deactivation of cationic CuI and AuI catalysts to form the corresponding inactive neutral chloride complexes [LMCl] (M=Cu or Au) can occur as a result of the generation of hydrogen chloride in the medium. This phenomenon was studied on the basis of a Mannich three-component coupling reaction (see scheme).
14 Jul 09:51
by Yahui Wang, Michael E. Muratore, Zhouting Rong, Antonio M. Echavarren
Abstract
7-Aryl-1,3,5-cycloheptatrienes react intermolecularly with methylenecyclopropanes in a triple gold(I)-catalyzed reaction to form cyclopentenes. The same formal (4+1) cycloaddition occurs with cyclobutenes. Other precursors of gold(I) carbenes can also be used as the C1 component of the cycloaddition.

Gold carbenes: 7-Aryl-1,3,5-cycloheptatrienes react intermolecularly with methylenecyclopropanes in a triple gold(I)-catalyzed reaction to form cyclopentenes. The same (4+1) cycloaddition occurs with cyclobutenes.
11 Jul 10:31
by Matthew Tredwell, Sean M. Preshlock, Nicholas J. Taylor, Stefan Gruber, Mickael Huiban, Jan Passchier, Joël Mercier, Christophe Génicot, Véronique Gouverneur
Abstract
Molecules labeled with fluorine-18 are used as radiotracers for positron emission tomography. An important challenge is the labeling of arenes not amenable to aromatic nucleophilic substitution (SNAr) with [18F]F−. In the ideal case, the 18F fluorination of these substrates would be performed through reaction of [18F]KF with shelf-stable readily available precursors using a broadly applicable method suitable for automation. Herein, we describe the realization of these requirements with the production of 18F arenes from pinacol-derived aryl boronic esters (arylBPin) upon treatment with [18F]KF/K222 and [Cu(OTf)2(py)4] (OTf=trifluoromethanesulfonate, py=pyridine). This method tolerates electron-poor and electron-rich arenes and various functional groups, and allows access to 6-[18F]fluoro-L-DOPA, 6-[18F]fluoro-m-tyrosine, and the translocator protein (TSPO) PET ligand [18F]DAA1106.

18F-labeling for PET: The nucleophilic 18F fluorination of pinacol-derived aryl boronic esters is achieved with [18F]KF/K222 in the presence of [Cu(OTf)2(py)4] (OTf=trifluoromethanesulfonate, py=pyridine); this unprecedented method can produce a clinical dose of 6-[18F]fluoro-L-DOPA in two steps (fluorination followed by deprotection) from a readily available shelf-stable arylBPin precursor (see scheme). RCY=decay-corrected radiochemical yield
11 Jul 09:08
by Hongchao Zheng, Laura L. Adduci, Ryan J. Felix, Michel R. Gagné
Abstract
An unprecedented gold-catalyzed diastereoselective cycloisomerization of 1,6-diynes bearing an alkylidene cyclopropane moiety has been developed. This methodology enables rapid access to a variety of 1,2-trimethylenenorbornanes, which are important building blocks in the preparations of abiotic and sesquiterpene core structures.

When gold met alkylidenecyclopropane: Cationic gold catalysts can mediate the highly exothermic (≈60 kcal mol−1) cycloisomerization of 1,6-diynes bearing an alkylidene cyclopropane moiety. This diastereoselective methodology efficiently generates 1,2-trimethylenenorbornanes, an important building block for abiotic targets and sesquiterpene natural products. DCE=1,2-dichloroethane, Tf=trifluoromethanesulfonyl, Tol=Tolyl.
11 Jul 08:54
by Matthew Tredwell, Sean M. Preshlock, Nicholas J. Taylor, Stefan Gruber, Mickael Huiban, Jan Passchier, Joël Mercier, Christophe Génicot, Véronique Gouverneur

Radiolabeling of arenes with [18F]fluoride by a transformational method using readily available aryl boronic ester precursors is reported by V. Gouverneur and co-workers in their Communication on page 7751 ff. The picture illustrates, in the confinement of a PET (positron emission tomography) scanner, the annihilation event resulting from positron emission from [18F]fluoro-L-DOPA.
11 Jul 08:06
by Yu Liu, Jia-Ling Zhang, Ren-Jie Song, Peng-Cheng Qian, Jin-Heng Li
Abstract
Here we describe the one-pot construction of the pyrrolo[4,3,2-de]quinolinone scaffold by a cascade nitration/cyclization sequence of 1,7-enynes with tBuONO and H2O. The cascade proceeds through alkene nitration, 1,7-enyne 6-exo-trig cyclization, C![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) H nitrations, and redox cyclization, and exhibits excellent functional group tolerance. The mechanism was investigated using in situ high-resolution mass spectrometry (HR-MS).
H nitrations, and redox cyclization, and exhibits excellent functional group tolerance. The mechanism was investigated using in situ high-resolution mass spectrometry (HR-MS).

Nitration cascade: The pyrrolo[4,3,2-de]quinolinone scaffold was synthesized by a metal-free reaction of N-(2-(ethynyl)aryl)acrylamides, tert-butyl nitrite and H2O. This cascade reaction is triggered by alkene nitration followed by 1,7-enyne 6-exo-trig cyclization, C![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) H nitrations, and redox cyclization and forms the product in good yields.
H nitrations, and redox cyclization and forms the product in good yields.
04 Jul 10:32
by Martín Fañanás-Mastral and Ben L. Feringa

Journal of the American Chemical Society
DOI: 10.1021/ja505281v

06 May 15:44
by Kazuhiro Takenaka, Suman C. Mohanta, Hiroaki Sasai
Abstract
A novel palladium-catalyzed reaction involving an unusual nucleophilic attack on a palladium enolate was developed using a spiro-bis(isoxazoline) (SPRIX) ligand. Treatment of alkynyl cyclohexadienone substrates with a Pd/SPRIX catalyst in acetic acid under an oxygen atmosphere furnished diacetoxylated benzofuranone derivatives in good yields. This cyclative diacetoxylation proceeded enantioselectively in the presence of an optically pure SPRIX ligand.

In a SPRIX: A new palladium-catalyzed reaction involving an unusual nucleophilic attack on a palladium enolate was developed using the SPRIX ligand. Treatment of alkynyl cyclohexadienone substrates with Pd/SPRIX in acetic acid under an oxygen atmosphere furnished diacetoxylated benzofuranone derivatives in good yields and with high enantioselectivity.
06 May 14:15
by Javier Carreras, Madeleine Livendahl, Paul R. McGonigal, Antonio M. Echavarren
Abstract
Three natural aromadendrane sesquiterpenes, (−)-epiglobulol, (−)-4β,7α-aromadendranediol, and (−)-4α,7α-aromadendranediol, have been synthesized in only seven steps in 12, 15, and 17 % overall yields, respectively, from (E,E)-farnesol by a stereodivergent gold(I)-catalyzed cascade reaction which forms the tricyclic aromadendrane core in a single step. These are the shortest total syntheses of these natural compounds.

Aromasynthesis: Aromadendrane sesquiterpenes (−)-epiglobulol, (−)-4α,7α-aromadendranediol, and (−)-4β,7α-aromadendranediol are synthesized in only seven steps from (E,E)-farnesol by a stereodivergent gold(I)-catalyzed cascade reaction.
01 May 09:21
by Valentina Bevilacqua, Mathias King, Manon Chaumontet, Marc Nothisen, Sandra Gabillet, David Buisson, Céline Puente, Alain Wagner, Frédéric Taran
Abstract
The concept of chelation-assisted copper catalysis was employed for the development of new azides that display unprecedented reactivity in the copper(I)-catalyzed azide–alkyne [3+2] cycloaddition (CuAAC) reaction. Azides that bear strong copper-chelating moieties were synthesized; these functional groups allow the formation of azide copper complexes that react almost instantaneously with alkynes under diluted conditions. Efficient ligation occurred at low concentration and in complex media with only one equivalent of copper, which improves the biocompatibility of the CuAAC reaction. Furthermore, such a click reaction allowed the localization of a bioactive compound inside living cells by fluorescence measurements.

Chelating azides were designed to form clickable copper complexes for efficient ligation with alkynes in complex biological media. Among a series of azides that bear nitrogen heterocycles, a bis(triazole) azide allowed ultra-fast click reactions with alkynes within seconds under diluted conditions. The reactivity and stability of this copper complex enabled efficient click reactions inside living cells.
26 Feb 18:55
by Chen Cheng
A catalyst that adds silyl groups to specific sites on aryl rings could streamline synthesis of pharmaceutical intermediates. [Also see Perspective by Tobisu and Chatani]
Authors: Chen Cheng, John F. Hartwig
26 Feb 12:03
by Richard A. Kerr
Researchers now have hard evidence that the circulation that helps warm the North Atlantic did indeed abruptly slow or perhaps even stop for centuries at a time more than 100,000 years ago.
Author: Richard A. Kerr
26 Feb 11:42
by Gretchen Vogel
An experimental technique that manipulates a woman's DNA could spare her from passing on a potentially deadly disease to her children, but the technique breaks new and ethically fraught ground.
Author: Gretchen Vogel
24 Feb 10:13
by Nicholas W. Mszar, Fredrik Haeffner and Amir H. Hoveyda

Journal of the American Chemical Society
DOI: 10.1021/ja500373s

24 Feb 09:27
by Peter I. O’Daniel, Zhihong Peng, Hualiang Pi, Sebastian A. Testero, Derong Ding, Edward Spink, Erika Leemans, Marc A. Boudreau, Takao Yamaguchi, Valerie A. Schroeder, William R. Wolter, Leticia I. Llarrull, Wei Song, Elena Lastochkin, Malika Kumarasiri, Nuno T. Antunes, Mana Espahbodi, Katerina Lichtenwalter, Mark A. Suckow, Sergei Vakulenko, Shahriar Mobashery and Mayland Chang

Journal of the American Chemical Society
DOI: 10.1021/ja500053x

No more posts. Check out what's trending.




![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) C bonds), uses commercially available reagents, and can be performed on a gram scale.
C bonds), uses commercially available reagents, and can be performed on a gram scale.
![[DOUBLE BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8fe.gif) C(C6F5)B(C6F5)2(CNtBu) with XeF2 gave Ph2P(F)C(p-Tol)
C(C6F5)B(C6F5)2(CNtBu) with XeF2 gave Ph2P(F)C(p-Tol)
















