Belén Rubial
Shared posts
A New Organocatalytic Concept for Asymmetric α-Alkylation of Aldehydes
Synthesis of Au(I) Trifluoromethyl Complexes. Oxidation to Au(III) and Reductive Elimination of Halotrifluoromethanes
The Role of Aryne Distortions, Steric Effects, and Charges in Regioselectivities of Aryne Reactions
[Report] Room-temperature enantioselective C–H iodination via kinetic resolution
Regioselective (Diacetoxyiodo)benzene-Promoted Halocyclization of Unfunctionalized Olefins
Gold-Catalyzed C–H Bond Functionalization of Metallocenes: Synthesis of Densely Functionalized Ferrocene Derivatives
Gold-Catalyzed Cycloisomerization of 1,6-Diyne Esters to 1H-Cyclopenta[b]naphthalenes, cis-Cyclopenten-2-yl δ-Diketones, and Bicyclo[3.2.0]hepta-1,5-dienes
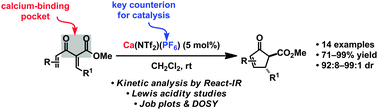
The first calcium-catalysed Nazarov cyclisation
DOI: 10.1039/C4CC06501H, Communication
The first calcium-catalysed Nazarov cyclisation is described.
The content of this RSS Feed (c) The Royal Society of Chemistry
Facile Oxidative Addition of Aryl Iodides to Gold(I) by Ligand Design: Bending Turns on Reactivity
Gold(I)-Catalyzed Highly Diastereo- and Enantioselective Alkyne Oxidation/Cyclopropanation of 1,6-Enynes
Abstract
A highly enantioselective oxidative cyclopropanation of 1,6-enynes catalyzed by cationic AuI/chiral phophoramidite complexes is presented. The new method provides convenient access to densely functionalized bicyclo[3.1.0]hexanes bearing three contiguous quaternary and tertiary stereogenic centers with high enantioselectivity (up to e.r. 98:2). Control experiments suggest that the quinoline moiety of the β-gold vinyloxyquinolinium intermediate in the reaction plays an important role in promoting good enantioselectivity through a transitional auxiliary effect in the transition state.

Playing it safe: A highly enantioselective oxidative cyclopropanation of 1,6-enynes with cationic AuI/chiral phosphoramidite catalysts provided convenient access to densely functionalized bicyclo[3.1.0]hexanes with three contiguous quaternary and tertiary stereogenic centers (see scheme; up to 92 % yield, e.r. 98:2). Control experiments suggest that the quinoline moiety in the oxidant plays an essential role in the enantioselective cyclopropanation.
Iodo Meyer–Schuster Rearrangement of 3-Alkoxy-2-yn-1-ols for β-Mono (Exclusively Z-Selective)-/Disubstituted α-Iodo-α,β-Unsaturated Esters
Dual Photoredox and Gold Catalysis: Intermolecular Multicomponent Oxyarylation of Alkenes
Abstract
Intermolecular three-component oxyarylation reactions of simple alkenes have been developed using a dual gold and photoredox catalytic system. Inexpensive organic dyes could be employed as the photocatalyst using aryldiazonium salts, while the combination of gold and iridium catalysts allowed for diaryliodonium compounds to be employed as the source of the arene coupling partner. In both cases, α-arylated ether products were generated under remarkably mild conditions using readily accessible visible light sources.

Axially Chiral Allenyl Gold Complexes
When gold meets chiral Bronsted acid catalysts: extending the boundaries of enantioselective gold catalysis
DOI: 10.1039/C4CC04633A, Feature Article
This review describes the development in the use of Au(I)/Bronsted acid binary catalytic systems to enable an enantioselective transformation in one-pot that cannot be achieved by gold catalysts alone.
The content of this RSS Feed (c) The Royal Society of Chemistry
Gold(I)-Catalyzed Furan-yne Cyclizations Involving 1,2-Rearrangement: Efficient Synthesis of Functionalized 1-Naphthols and Its Application to the Synthesis of Wailupemycin G
Abstract
Gold-catalyzed cascade cyclization/1,2-rearrangement of 1-(2-furanyl)phenyl propargyl alcohols has been developed, which provides a rapid and efficient access to multisubstituted 1-naphthols bearing an enal or enone moiety with high stereoselectivity. The (Z)- or (E)-stereochemistry can be easily controlled by choosing protected- or non-protected substrates. The utility of the methodology has been illustrated in the first total synthesis of wailupemycin G.

Rearrangement: Gold-catalyzed cascade cyclization/1,2-rearrangement of 1-(2-furanyl)phenyl propargyl alcohols has been developed, which provides a rapid and efficient access to multisubstituted 1-naphthols bearing an enal or enone moiety with high stereoselectivity. The (Z)- or (E)-stereochemistry can be easily controlled by choosing protected- or non-protected substrates. The utility of the methodology has been illustrated in the first total synthesis of wailupemycin G (see scheme).
Gold-Catalyzed Cycloisomerization of 1,6,8-Dienyne Carbonates and Esters to cis-Cyclohepta-4,8-diene-Fused Pyrrolidines
Abstract
A synthetic approach that provides access to cis-cyclohepta-4,8-diene-fused pyrrolidines efficiently through AuI-catalyzed cycloisomerization of 1,6,8-dienyne carbonates and esters at a low catalyst loading of 2 mol % is reported. Starting carbonates and esters with a pendant alkyl group on the terminal alkenyl carbon center were found to favor tandem 1,2-acyloxy migration/cyclopropanation followed by Cope rearrangement of the resulting cis-3-azabicyclo[3.1.0]hexane intermediate. On the other hand, substrates containing a terminal diene or starting materials in which the distal alkene moiety bears a phenyl substituent were observed to undergo competitive but reversible 1,3-acyloxy migration prior to the nitrogen-containing bicyclic ring formation. The delineated reaction mechanism also provides experimental evidence for the reversible interconversion between the oft-proposed organogold intermediates obtained in this step of the tandem process.

Paths of gold: A synthetic method to prepare cis-cyclohepta-4,8-diene-fused pyrrolidines efficiently through AuI-catalyzed cycloisomerization of 1,6,8-dienyne carbonates and esters at a low catalyst loading (2 mol %) is reported (see scheme). A study focusing on delineating the reaction mechanism provided experimental evidence of the reversibility of the [2,3]- and [3,3]-sigmatropic rearrangement pathways, which have often been proposed but rarely supported by direct experimental evidence in gold catalysis.
Enantioselective Synthesis of Highly Functionalized Dihydrofurans through Copper-Catalyzed Asymmetric Formal [3+2] Cycloaddition of β-Ketoesters with Propargylic Esters
Abstract
An enantioselective synthesis of highly functionalized dihydrofurans through a copper-catalyzed asymmetric [3+2] cycloaddition of β-ketoesters with propargylic esters has been developed. With a combination of Cu(OTf)2 and a chiral tridentate P,N,N ligand as the catalyst, a variety of 2,3-dihydrofurans bearing an exocyclic double bond at the 2 position were obtained in good chemical yields and with good to high enantioselectivities. The exocyclic double bond can be hydrogenated in a highly diastereoselective fashion to give unusual cis-2,3-dihydrofuran derivatives, thus further enhancing the scope of this transformation.

The combination of Cu(OTf)2 and a chiral tridentate P,N,N ligand as the catalyst enabled the title reaction, giving a variety of 2-methylene-2,3-dihydrofurans in good yields and with good to high enantioselectivities. Furthermore, the exocyclic methylene group can be hydrogenated in a highly diastereoselective fashion to give unusual cis-2,3-dihydrofuran derivatives.
Asymmetric Gold-Catalyzed Lactonizations in Water at Room Temperature
Abstract
Asymmetric gold-catalyzed hydrocarboxylations are reported that show broad substrate scope. The hydrophobic effect associated with in situ-formed aqueous nanomicelles gives good to excellent ee’s of product lactones. In-flask product isolation, along with the recycling of the catalyst and the reaction medium, are combined to arrive at an especially environmentally friendly process.

With tight quarters come more interactions! The high concentrations within nanomicelles can be used to force a close interaction between a nonracemic cationic metal complex and its nonracemic counterion, thereby maximizing the catalyst's impact on a given transformation. A chiral gold dicationic species and its associated phosphate anion are used to control absolute stereochemistry in newly formed γ-lactone products, derived from allenic carboxylic acids.
Gold(I)-Catalyzed Diazo Coupling: Strategy towards Alkene Formation and Tandem Benzannulation
Abstract
A gold(I)-catalyzed cross-coupling of diazo compounds to afford tetrasubstituted alkenes has been developed by taking advantage of a trivial electronic difference between two diazo substrates. A N-heterocyclic-carbene-derived gold complex is the most effective catalyst for this transformation. Based on this new strategy, a gold(I)-initiated benzannulation has been achieved through a tandem reaction involving a diazo cross-coupling, 6π electrocyclization, and oxidative aromatization.

Crossing paths: A gold(I)-catalyzed cross-coupling of diazo compounds to afford tetrasubstituted alkenes has been developed. In addition, a gold(I)-initiated benzannulation has been achieved in a tandem reaction involving cross-coupling of the vinyldiazoacetates, sequential 6π electrocyclization, and oxidative aromatization.
The Au(I) Catalyzed Activation of Allenamides and Their Subsequent Transformation into Chromanes: A Method for the Regiocontrolled Addition to the α- and γ-Positions of the Allene Unit
The Chemical Bond in Gold(I) Complexes with N-Heterocyclic Carbenes
Reactivity of Organogold Compounds with B(C6F5)3: Gold–Boron Transmetalation via σ-B/π-Au Species
[Au]/[Pd] Multicatalytic Processes: Direct One-Pot Access to Benzo[c]chromenes and Benzo[b]furans
Abstract
A new synthetic protocol that combines the advantages offered by eco-friendly solvent-free reactions and sequential transformations is reported. This strategy offers straightforward access to benzo[c]chromenes and benzo[b]furans from commercially available starting materials. This two-step, one-pot strategy consists of an Au-catalyzed hydrophenoxylation process followed by Pd-catalyzed C![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) H activation or Mizoroki–Heck reactions. The selectivity of the process towards C
H activation or Mizoroki–Heck reactions. The selectivity of the process towards C![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) H activation or Mizoroki–Heck reaction can be easily tuned.
H activation or Mizoroki–Heck reaction can be easily tuned.

Very neat reactions! A new strategy for the synthesis of benzo[c]chromenes and benzo[b]furans from commercially available starting materials is reported. This two-step, one-pot strategy consists in a gold-catalyzed hydrophenoxylation reaction followed by Pd-catalyzed C![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) H activation or Mizoroki–Heck reactions.
H activation or Mizoroki–Heck reactions.
Role of Gold(I) α-Oxo Carbenes in the Oxidation Reactions of Alkynes Catalyzed by Gold(I) Complexes
[Report] Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO2
Gold-catalyzed intermolecular oxidation of o-alkynylbiaryls: an easy and practical access to functionalized fluorenes
DOI: 10.1039/C4CC05115G, Communication
A novel gold-catalyzed intermolecular oxidation of o-alkynylbiaryls has been developed. A variety of functionalized fluorenes are readily accessed by utilizing this non-diazo approach, thus providing a viable alternative to synthetically useful fluorenes.
The content of this RSS Feed (c) The Royal Society of Chemistry
Stereoselective Construction of 1,3-Disilylcyclopentane Derivatives by Scandium-Catalyzed [3+2] Cycloaddition of Allylsilanes to β-Silylenones
Abstract
The Sc(OTf)3-catalyzed [3+2] cycloaddition of allylsilanes to β-silyl-α,β-unsaturated ketones (β-silylenones) has been developed to form five-membered syn-1,3-disilylketones diastereoselectively through the rearrangement of the silicon substituents on the allylsilane. Stabilization of the carbocation intermediates by a double silicon effect plays a key role in directing the course of the reaction to favor the [3+2] cycloaddition pathway over simple allylation.

Two silicon atoms collaborate: The Sc(OTf)3-catalyzed stereoselective [3+2] cycloaddition of allylsilanes to β-silylenones with migration of one silyl group has been developed. The electronic effect of two silyl groups plays a key role for selective cycloaddition. Two silyl groups of the obtained cycloadduct can be regioselectively oxidized into hydroxy groups. X-ray crystal structure: C gray, H white, Si yellow, O red.
Chemoselective Carbophilic Addition of α-Diazoesters through Ligand-Controlled Gold Catalysis
Abstract
The chemoselective addition of arenes and 1,3-diketones to α-aryldiazoesters was achieved through ligand-controlled gold catalysis. Unlike a dirhodium catalyst (which promotes C
![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) H insertion and cyclopropanation) and a copper catalyst (which catalyzes O
H insertion and cyclopropanation) and a copper catalyst (which catalyzes O![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) H and N
H and N![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) H insertions), the gold catalyst with an electron-deficient phosphite as the ancillary ligand exclusively gave the carbophilic addition product, thus representing a new and efficient approach to form “carbophilic carbocations”, which selectively react with carbon nucleophiles.
H insertions), the gold catalyst with an electron-deficient phosphite as the ancillary ligand exclusively gave the carbophilic addition product, thus representing a new and efficient approach to form “carbophilic carbocations”, which selectively react with carbon nucleophiles.

Carbocation or carbene? The chemoselective addition of arenes and 1,3-diketones to α-aryldiazoesters was achieved through ligand-controlled gold catalysis. The gold catalyst with electron-deficient phosphite as the ancillary ligand exclusively gave the carbophilic addition products, thus representing a new and efficient approach to form “carbophilic carbocations”, which selectively react with carbon nucleophiles.
Gold-Catalyzed Oxa-Povarov Reactions for the Synthesis of Highly Substituted Dihydrobenzopyrans from Diaryloxymethylarenes and Olefins
Abstract
Oxa-Povarov reactions involving readily available diaryloxymethylarenes and aryl-substituted alkenes are reported. Their [4+2] cycloadditions were efficiently catalyzed by IPrAuSbF6 (IPr=1,3-bis(diisopropylphenyl)imidazol-2-ylidene) with high diastereoselectivity. Product analysis revealed that the reactions likely proceed by a stepwise ionic mechanism, because both E- and Z-configured β-methylstyrene gave the same cycloadducts in the same proportions.

Stepwise ionic mechanism: Oxa-Povarov reactions involving readily available diaryloxymethylarenes and aryl-substituted alkenes are reported. Their [4+2] cycloadditions were efficiently catalyzed by IPrAuSbF6 with high diastereoselectivity (see scheme, IPr=1,3-bis(diisopropylphenyl)imidazol-2-ylidene). Product analysis revealed that the reactions likely proceed by a stepwise ionic mechanism, because both E- and Z-configured β-methylstyrene gave the same cycloadducts in the same proportions.