07 May 17:38
Chem. Commun., 2015, 51,8730-8741
DOI: 10.1039/C5CC00120J, Feature Article
Justin A. Goodwin, Aaron Aponick
The issue of regioselectivity in the Au-catalyzed hydration and hydroalkoxylation of alkynes is a perpetual challenge that essentially remains an unsolved problem with respect to general solutions.
The content of this RSS Feed (c) The Royal Society of Chemistry

06 May 09:31
by Mattia Silvi, Elena Arceo, Igor D. Jurberg, Carlo Cassani and Paolo Melchiorre

Journal of the American Chemical Society
DOI: 10.1021/jacs.5b01662

17 Apr 10:26
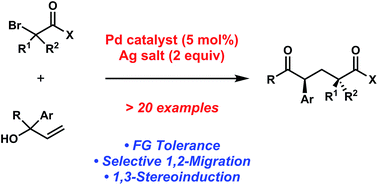
Chem. Sci., 2015, 6,2777-2781
DOI: 10.1039/C5SC00505A, Edge Article

Open Access
Yang Yu, Uttam K. Tambar
A palladium-catalyzed coupling of [small alpha]-bromocarbonyl compounds and allylic alcohols has been developed for the generation of acyclic aryl-substituted dicarbonyl compounds.
The content of this RSS Feed (c) The Royal Society of Chemistry

17 Apr 10:14
by Naganaboina Naveen, Srinivasa Rao Koppolu, Rengarajan Balamurugan
Abstract
A practically simple and direct α-alkylation of unactivated ketones using benzylic alcohols has been achieved. The in situ formed acetals are the key for the success of the reaction. The catalyst, silver hexafluoroantimonate(V) (AgSbF6) provides double activation by converting the ketone into an enol ether via acetal and generation of carbocationic center at the benzylic position of the benzylic alcohol. The alcohols include benzylic propargyl alcohols, cinnamyl alcohols, and diarylmethanols.

04 Mar 19:59
by Bradley D. Robertson, Rachel E. M. Brooner, Ross A. Widenhoefer
Abstract
Mixtures of [{PCy2(o-biphenyl)}AuCl] and AgSbF6 catalyze the tandem cycloaddition/hydroarylation of 7-aryl-1,6-enynes with electron-rich arenes to form 6,6-diarylbicyclo[3.2.0]heptanes in good yield under mild conditions. Experimental observations point to a mechanism involving gold-catalyzed cycloaddition followed by silver-catalyzed hydroarylation of a bicyclo[3.2.0]hept-1(7)-ene intermediate.

Gold first, silver second: Mixtures of [{PCy2(o-biphenyl)}AuCl] and AgSbF6 catalyze the tandem cycloaddition/hydroarylation of 7-aryl-1,6-enynes with electron-rich arenes to form 6,6-diarylbicyclo[3.2.0]heptanes in good yield under mild conditions. Experimental observations point to a mechanism involving a gold-catalyzed cycloaddition followed by a silver-catalyzed hydroarylation of the bicyclo[3.2.0]hept-1(7)-ene intermediate.
02 Mar 16:00
by Kaitlyn M. Logan, Kevin B. Smith, M. Kevin Brown
Abstract
A method for the diastereoselective carboboration of 1,2-disubstituted styrenes with aryl/vinyl bromides and (Bpin)2 is reported. High diastereoselectivities and yields are observed for the formation of either diastereomer of the product from a single alkene isomer. These reactions provide access to a diverse range of structures from simple starting materials.

The diastereoselective carboboration of 1,2-disubstituted styrenes with aryl/vinyl bromides and (Bpin)2 proceeds with high diastereoselectivities and yields. The formation of either diastereomer of the product from a single alkene isomer is determined by the reaction conditions. The method thus provides access to a diverse range of structures from simple starting materials.
02 Mar 15:50
Chem. Sci., 2015, 6,2903-2908
DOI: 10.1039/C5SC00295H, Edge Article

Open Access
Helio Faustino, Ivan Varela, Jose L. Mascarenas, Fernando Lopez
A novel fully intermolecular gold-catalyzed [2 + 2 + 2] cycloaddition involving an allenamide, an alkene and an aldehyde provides a straightforward entry to tetrahydropyrans.
The content of this RSS Feed (c) The Royal Society of Chemistry

20 Feb 13:28
by Danila Gasperini, Alba Collado, Adrián Goméz-Suárez, David B. Cordes, Alexandra M. Z. Slawin, Steven P. Nolan
Abstract
A new synthetic strategy was devised leading to the formation of complexes, such as [Au(IPr)(CH2COCH3)]. The approach capitalizes on the formation of a decomposition product observed in the course of the synthesis of [Au(IPr)(Cl)]. A library of gold acetonyl complexes containing the most common N-heterocyclic carbene (NHC) ligands has been synthesized. These acetonyl complexes are good synthons for the preparation of numerous organogold complexes. Moreover, they have proven to be precatalysts in common gold(I)-catalyzed reactions.

Organogold: A library of [Au(NHC)(acetonyl)] complexes (NHC=N-heterocyclic carbene) has been easily synthesized. These complexes represent valuable synthetic precursors to a plethora of organogold species, as well as active catalysts for gold(I)-catalyzed reactions (see scheme).
16 Feb 20:01
by Barry M. Trost, Etienne J. Donckele, David A. Thaisrivongs, Maksim Osipov and James T. Masters

Journal of the American Chemical Society
DOI: 10.1021/jacs.5b00786

13 Feb 11:04
by Anat Milo
Knowledge of chemical reaction mechanisms can facilitate catalyst optimization, but extracting that knowledge from a complex system is often challenging. Here, we present a data-intensive method for deriving and then predictively applying a mechanistic model of an enantioselective organic reaction. As a validating case study, we selected an intramolecular dehydrogenative C-N coupling reaction, catalyzed by chiral phosphoric acid derivatives, in which catalyst-substrate association involves weak, noncovalent interactions. Little was previously understood regarding the structural origin of enantioselectivity in this system. Catalyst and substrate substituent effects were probed by means of systematic physical organic trend analysis. Plausible interactions between the substrate and catalyst that govern enantioselectivity were identified and supported experimentally, indicating that such an approach can afford an efficient means of leveraging mechanistic insight so as to optimize catalyst design.
Authors: Anat Milo, Andrew J. Neel, F. Dean Toste, Matthew S. Sigman
10 Feb 13:07
by Steffen Mader, Lise Molinari, Matthias Rudolph, Frank Rominger , A. Stephen K. Hashmi
Abstract
Various haloalkynes are converted in the presence of a dual activation gold catalyst. Via a dual activation process a completely atom economic head-to-tail coupling delivers gem-dihalogenated conjugated enynes as valuable building blocks for organic synthesis.

Various haloalkynes are converted in the presence of a dual-activation gold catalyst. Through a dual activation process, a completely atom-economic head-to-tail coupling delivers gem- dihalogenated conjugated enynes as valuable building blocks for organic synthesis.
10 Feb 12:54
by Jin-Ming Yang, Xiang-Ying Tang, Min Shi
Abstract
A gold-catalyzed intramolecular cycloisomerization of α-yne-furans 1 is described in this contribution. A variety of cyclic α,β-unsaturated aldehyde or ketone derivatives and nitrogen-containing tricyclic adducts were obtained selectively in moderate to excellent yields under mild conditions by varying the substituents on the standard substrates.

Going for gold: A variety of cyclic α,β-unsaturated aldehyde or ketone derivatives and tricyclic adducts were obtained selectively through a gold-catalyzed intramolecular reaction of propargylic esters with furan rings (see scheme). Several interesting transformations of the products are also reported.
10 Feb 11:22
by Christopher B. Kelly, Kyle M. Lambert, Michael A. Mercadante, John M. Ovian, William F. Bailey, Nicholas E. Leadbeater
Abstract
A scalable, high yielding, rapid route to access an array of nitriles from aldehydes mediated by an oxoammonium salt (4-acetylamino-2,2,6,6-tetramethylpiperidine-1-oxoammonium tetrafluoroborate) and hexamethyldisilazane (HMDS) as an ammonia surrogate has been developed. The reaction likely involves two distinct chemical transformations: reversible silyl-imine formation between HMDS and an aldehyde, followed by oxidation mediated by the oxoammonium salt and desilylation to furnish a nitrile. The spent oxidant can be easily recovered and used to regenerate the oxoammonium salt oxidant.

A serendipitous find: The scalable and high yielding title reaction is mediated by 4-acetylamino-2,2,6,6-tetramethylpiperidine-1-oxoammonium tetrafluoroborate and hexamethyldisilazane (HMDS). The reaction likely involves reversible silyl-imine formation between HMDS and an aldehyde, and subsequent oxidation and desilylation. The spent oxidant can be easily recycled.
06 Feb 19:06
by Veluru Ramesh Naidu, Juho Bah, Johan Franzén
Abstract
A direct organocatalytic carbonyl/olefin oxo-metathesis has been developed. The reaction is catalyzed by trityl tetrafluoroborate (TrBF4) and utilizes unactivated alkenes for the olefination of aromatic aldehydes to give trans β-alkylstyrenes in yields of 44–85 % with only acetone as the byproduct. The pronounced Lewis acidity of the carbocation results in unusual reactivity that is proposed to catalyze a stepwise [2+2] cycloaddition to give an oxetane intermediate. Fragmentation of the latter in a formal retro [2+2] reaction gives the oxo-metathesis product.

A new partner in the metathesis dance: Trityl tetrafluoroborate (TrBF4) catalyzes the direct oxo-metathesis of aldehydes and unactivated olefins to give β-alkylstyrene derivatives and acetone through an unusual metal-free formal [2+2]/retro [2+2] reaction sequence.
06 Feb 19:05
by Sinan Wang, Xiangmin Li, Hongwei Liu, Li Xu, Jinchen Zhuang, Jian Li, Hao Li and Wei Wang

Journal of the American Chemical Society
DOI: 10.1021/ja511143b

06 Feb 19:03
by Tobias Lauterbach, Takafumi Higuchi, Matthias W. Hussong, Matthias Rudolph, Frank Rominger, Kazushi Mashima, A. Stephen K. Hashmi
Abstract
Aromatic diyne systems bearing one terminal propargylic acetate moiety and one tertiary propargylic alcohol subunit were converted in the presence of a gold catalyst. After an initial 1,2-migration of the acetoxy group at the terminal alkyne, a gold carbenoid is formed which is then transferred onto the internal alkyne of the propargylic alcohol. This combination enables the use of propargylic acetates as precursors for a gold-catalyzed pinacol-type rearrangement. In the final pinacol-like step the shift of an alkyl or aryl moiety onto an electrophilic gold carbenoid/cation terminates the reaction and 1-naphthyl ketones are obtained as products. If electron-rich aromatic backbones are used, a mechanistically interesting alternative pathway including a retro-Buchner reaction is opened.

03 Feb 16:07
by Kelvin S. L. Chan, Hai-Yan Fu and Jin-Quan Yu

Journal of the American Chemical Society
DOI: 10.1021/ja512529e

23 Jan 10:17
by Yusuke Tokimizu, Marcel Wieteck, Matthias Rudolph, Shinya Oishi, Nobutaka Fujii, A. Stephen K. Hashmi and Hiroaki Ohno

Organic Letters
DOI: 10.1021/ol503623m

09 Jan 09:26
by Pascal Nösel, Vanessa Müller, Steffen Mader, Setareh Moghimi, Matthias Rudolph, Ingo Braun, Frank Rominger, A. Stephen K. Hashmi
Abstract
1,5-Diynes bearing halogen-substituted alkynes were synthesized and converted in the presence of a gold catalyst. In contrast to the corresponding hydroarylating aromatization reaction with terminal alkynes, a totally different reaction mode was observed. Instead of the expected dual catalysis pathway, only one gold center is needed and a 1,2-halogen migration is initiated in which either a gold vinylidene species or a gold carbenoid is involved. By the incorporation of one solvent molecule, diiodinated aromatic products are obtained in high selectivity.

10 Dec 13:06
by Charlotte G. Watson, Angelica Balanta, Tim G. Elford, Stéphanie Essafi, Jeremy N. Harvey and Varinder K. Aggarwal

Journal of the American Chemical Society
DOI: 10.1021/ja509029h

03 Dec 18:55
Chem. Commun., 2015, 51,889-891
DOI: 10.1039/C3CC48897G, Communication
Yinliang Guo, Qiang Liu, Yanxing Jia
A concise synthesis of the tricyclic skeleton of crotobarin and crotogoudin via a gold-catalyzed 1,6-enyne cycloisomerization reaction is reported.
The content of this RSS Feed (c) The Royal Society of Chemistry

03 Dec 18:55
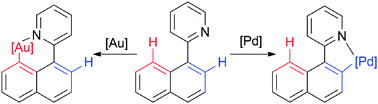
Chem. Commun., 2015, 51,911-913
DOI: 10.1039/C4CC07962K, Communication
Mikhail Kondrashov, Sudarkodi Raman, Ola F. Wendt
Cyclometallations of 2-(1-naphthyl)-pyridine with gold and palladium salts proceed with completely orthogonal regioselectivities.
The content of this RSS Feed (c) The Royal Society of Chemistry

03 Dec 18:54
Chem. Commun., 2015, 51,5322-5325
DOI: 10.1039/C4CC08520E, Communication
M. C. Holland, J. B. Metternich, C. Muck-Lichtenfeld, R. Gilmour
A cation-[small pi] interaction is operational in the addition of uncharged nucleophiles to iminium salts derived from MacMillan's 1st generation catalyst.
The content of this RSS Feed (c) The Royal Society of Chemistry

03 Dec 16:54
by Christopher A. Swift and Scott Gronert

Organometallics
DOI: 10.1021/om500926v

27 Nov 19:13
by Juho Bah, Veluru Ramesh Naidu, Johannes Teske, Johan Franzén
Abstract
One class of potential Lewis acids that has received negligible attention as a catalyst is the carbocation. Here we show the potential of triarylmethylium ions as highly powerful Lewis acid catalysts for organic reactions. The Lewis acidity of the triarylmethylium ion can be easily tuned by variation of the electronic properties of the aromatic rings and the catalytic activity of the carbocation is shown to correlate directly to the level of stabilization of the empty pC-orbital at the cationic carbon. The versatility of triarylmethylium ions as efficient Lewis acid catalysts for organic reactions is demonstrated in Diels–Alder, aza-Diels–Alder, conjugate addition, halogenation, epoxide rearrangement and intramolecular hetro-ene reactions.

25 Nov 20:22
by Sang Min Kim, Dabon Lee and Soon Hyeok Hong

Organic Letters
DOI: 10.1021/ol503055x

11 Nov 12:00
Chem. Sci., 2014, Advance Article
DOI: 10.1039/C4SC02148G, Edge Article
Ayan Maity, Amanda N. Sulicz, Nihal Deligonul, Matthias Zeller, Allen D. Hunter, Thomas G. Gray
Cyclometalated gold(III) aryls are prepared through palladium catalysis. Mono- and diarylation are demonstrated. A wide range of functional groups is tolerated.
To cite this article before page numbers are assigned, use the DOI form of citation above.
The content of this RSS Feed (c) The Royal Society of Chemistry

06 Nov 16:23
by Bo Han, Xin Xie, Wei Huang, Xiang Li, Lei Yang, Cheng Peng
Abstract
Modulating the diastereoselectivity of stereochemically complex molecules in asymmetric synthesis remains a challenge, especially when the reaction cycle produces no net change in the catalysts, ligands, substrates or additives. Here we accomplish a controlled diastereodivergence in the asymmetric synthesis of fully functionalized tetrahydropyrans by adjusting the sequence of an organocatalytic cascade reaction. We also show that this one-pot operation provides two synthetically important architectures with excellent stereocontrol. To the best of our knowledge, this is the first published kinetic resolution of MBH alcohols in an organocascade.

06 Nov 16:23
by Luis M. Castelló, Carmen Nájera, José M. Sansano, Olatz Larrañaga, Abel de Cózar, Fernando P. Cossío
Abstract
Chiral complexes formed by privileged phosphoramidites and silver triflate or silver benzoate are excellent catalysts for the general 1,3-dipolar cycloaddition between azomethine ylides generated from α-amino acid-derived imino esters and nitroalkenes affording with high dr the exo-cycloadducts 4,5-trans-2,5-cis-4-nitroprolinates in high ee at room temperature. In general, better results are obtained using silver rather than copper(II) complexes. In many cases the exo-cycloadducts can be obtained in enantiomerically pure form just after simple recrystallization. The mechanism and the justification of the experimentally observed stereodiscrimination of the process are supported by DFT calculations. These enantiomerically enriched exo-nitroprolinates can be used as reagents for the synthesis of nitropiperidines, by ester reduction and ring expansion, which are inhibitors of farnesyltransferase.

06 Nov 16:22
Chem. Commun., 2014, Accepted Manuscript
DOI: 10.1039/C4CC08174A, Communication
S. S. V. Ramasastry, Seema Dhiman
An efficient relay catalytic process involving Au(I)/Bronsted acid to access various polysubstituted cyclopentannulated indoles from easily accessible 1-(2-aminophenyl)prop-2-ynols and readily available 1,3-dicarbonyls has been developed. In an unprecedented event, the...
The content of this RSS Feed (c) The Royal Society of Chemistry


 Open Access
Open Access