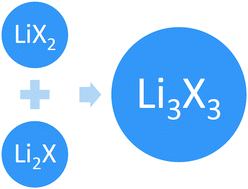Shared posts
[ASAP] Nickel-Catalyzed Isomerization/Allylic Cyanation of Alkenyl Alcohols
6‐Arylphenanthridines from Aryl o‐Biaryl Ketones with 1,1,1,3,3,3‐Hexamethyldisilazane and Molecular Iodine

Aryl biaryl ketones were transformed into 6‐arylphenanthridines efficiently by the reaction with 1,1,1,3,3,3‐hexamethyldisilazane in the presence of Sc(OTf)3 in toluene, followed by removal of the solvent, and the subsequent reaction with molecular iodine and K2CO3 in a mixture of THF and methanol.
Warming treatment of aryl o‐biaryl ketones with 1,1,1,3,3,3‐hexamethyldisilazane in the presence of Sc(OTf)3 in toluene, followed by the reaction with molecular iodine and K2CO3 in a mixture of THF and methanol at 60 °C gave the corresponding 6‐arylphenanthridines in good to moderate yields. The present reaction is a one‐pot method for the preparation of 6‐arylphenanthridines from aryl o‐biaryl ketones through the cyclization of imino‐nitrogen‐centered radicals that were generated from N‐iodo aryl o‐biaryl ketimines formed from the reaction of aryl biaryl ketimines with molecular iodine.
[ASAP] Enantioselective Synthesis of Axially Chiral Biaryls via Cu-Catalyzed Acyloxylation of Cyclic Diaryliodonium Salts

Selective Debromination and α-Hydroxylation of α-Bromo Ketones Using Hantzsch Esters as Photoreductants
Abstract
Two transformations initiated by photoinduced one-electron transfer to α-bromo ketones have been demonstrated. Hantzsch esters donate one electron to α-bromo ketones under photoirradiation, promoting reductive debromination. Subsequent reactions of the resulting radical species of the ketones with molecular oxygen and Hantzsch esters lead to α-hydroxylation or debromination, respectively. The relative dominance of the two pathways depends profoundly on the reaction conditions, including solvent, O2 levels, and the concentration of the Hantzsch esters. The synthetic protocols feature advantages because they require the environmentally benign sources, molecular oxygen and visible light.

Visible‐Light Promoted Polycyclizations of Dienynes
A variety of linear dienynes can deliver complex tetracyclic frameworks in the presence of an Ir(III) complex and visible light. Products feature the assembly of four new C‐C bonds and six contiguous stereocenters, which decorate two [3.1.0] bicyclic units tethered through their bridging quaternary carbons. The internal alkyne acts as a formal dicarbenoid to assemble two cyclopropanes in these radical cationic cascades. This behavior has not been previously observed among organic reactive intermediates and can be extended to intermolecular reactions and diendiynes.
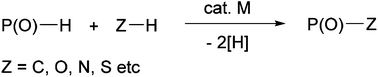
Dehydrogenative coupling involving P(O)-H bonds: a powerful way for the preparation of phosphoryl compounds
漂流NBB!
DOI: 10.1039/C5DT01896J, Frontier
This Frontier highlights the recent progress in the preparation of organophosphorus compounds via transition metal-catalysed dehydrogenative couplings of P(O)H compounds with Z-H compounds.
To cite this article before page numbers are assigned, use the DOI form of citation above.
The content of this RSS Feed (c) The Royal Society of Chemistry
Palladium-Catalyzed α,β-Dehydrogenation of Esters and Nitriles
漂流C-C 脱氢 C=C, XJ!!!
Mn-Catalyzed Highly Efficient Aerobic Oxidative Hydroxyazidation of Olefins: A Direct Approach to β-Azido Alcohols
漂流I2 Cat. 的CC情何以堪!
Decarboxylative Alkynyl Termination of Palladium-Catalyzed Catellani Reaction: A Facile Synthesis of α-Alkynyl Anilines via Ortho C–H Amination and Alkynylation
漂流我靠!不换N,只能发到OL上了!
Reduction of phosphine oxides to the corresponding phosphine derivatives in Mg/Me3SiCl/DMI system
Source:Tetrahedron Letters, Volume 56, Issue 7
Author(s): Manabu Kuroboshi , Toshihito Kita , Asuka Aono , Toshimasa Katagiri , Seiya Kikuchi , Syoko Yamane , Hiromu Kawakubo , Hideo Tanaka
Direct reductions of phosphine oxides to the corresponding phosphines were performed successfully by using Mg/Me3SiCl/DMI system. The reduction proceeded under mild conditions and was applicable to a wide range of phosphine oxides; triarylphosphine oxides, alkyldiarylphosphine oxides, and dialkylarylphosphine oxides gave the corresponding phosphines in good to excellent yields.
Graphical abstract

Iridium(III)-Catalyzed CH Amidation of Arylphosphoryls Leading to a P-Stereogenic Center
Abstract
Direct C![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) H amidation of arylphosphoryl compounds has been developed by using an IrIII catalyst system under mild conditions. A wide range of substrates could be employed with high functional-group tolerance. This procedure was successfully applied for the first time to the asymmetric reaction giving rise to a P-chirogenic center with a high diastereomeric ratio of up to 19:1 (90 % de).
H amidation of arylphosphoryl compounds has been developed by using an IrIII catalyst system under mild conditions. A wide range of substrates could be employed with high functional-group tolerance. This procedure was successfully applied for the first time to the asymmetric reaction giving rise to a P-chirogenic center with a high diastereomeric ratio of up to 19:1 (90 % de).

A road to P-chirality: A cationic [IrIII(Cp*)] complex catalyzes the direct C![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) H amidation of diarylphosphorus compounds bearing a chiral auxiliary to allow for a straightforward route to optically pure P-chiral compounds (see scheme; Cp*=1,2,3,4,5-pentamethylcyclopentadiene).
H amidation of diarylphosphorus compounds bearing a chiral auxiliary to allow for a straightforward route to optically pure P-chiral compounds (see scheme; Cp*=1,2,3,4,5-pentamethylcyclopentadiene).
DFT Studies on the Silver-Catalyzed Carboxylation of Terminal Alkynes with CO2: An Insight into the Catalytically Active Species
Synthesis of Fluorinated Heteroaromatics through Formal Substitution of a Nitro Group by Fluorine under Transition-Metal-Free Conditions
Abstract
An efficient and transition-metal-free approach was developed to access a series of fluorinated heteroaromatics in moderate to excellent yields. This one-pot procedure features a triple-relay transformation of rapid dearomatization, fluorination, and rearomatization processes, which represents a conceptually novel strategy of combining partial hydrogenation and electrophilic fluorination.

An efficient and transition-metal-free approach was developed to access a series of fluorinated heteroaromatics in moderate to excellent yields through formal substitution of a nitro group by fluorine (see scheme). This one-pot procedure features a triple-relay transformation of rapid dearomatization, fluorination, and rearomatization processes, which represents a conceptually novel strategy of combining partial hydrogenation and electrophilic fluorination.
One-Pot Transformation of Methylarenes into Aromatic Nitriles with Inorganic Metal-Free Reagents
漂流米兰城从来不缺少英雄!
Abstract
Various methylarenes were transformed into the corresponding aromatic nitriles in good to moderate yields by the treatment with aq. HBr and aq. H2O2, followed by reaction with molecular iodine and aq. ammonia in a one-pot procedure. The present reaction is a useful, practical, transition-metal-free, and organic-reagent-free method for the preparation of aromatic nitriles from methylarenes.

Various methylarenes were treated with aq. HBr and aq. H2O2 under warming conditions and/or irradiation conditions, followed by the reaction with molecular iodine and aq. ammonia, to provide the corresponding aromatic nitriles, in a one-pot procedure. The present reaction was carried out under metal-free and organic-reagent-free conditions.
Novel Li3X3 supersalts (X = F, Cl, Br & I) and their alkalide characteristics
DOI: 10.1039/C3NJ01600E, Paper
This interaction is stronger than that between Li and X, forming traditional LiX salts. Thus, these non-traditional Li3X3 species should be regarded as supersalts which consist of Li2X and LiX2 superatomic moieties.
The content of this RSS Feed (c) The Royal Society of Chemistry