Shared posts
3-(Piperidin-4-ylmethoxy)pyridine Containing Compounds Are Potent Inhibitors of Lysine Specific Demethylase 1
MChemLSD1 inhibitors
T3P-Promoted, Mild, One-Pot Syntheses of Constrained Polycyclic Lactam Dipeptide Analogues via Stereoselective Pictet–Spengler and Meyers Lactamization Reactions
Evaluation of tert-Butyl Isosteres: Case Studies of Physicochemical and Pharmacokinetic Properties, Efficacies, and Activities
MChembioisoteric replacement
Abstract
The tert-butyl group is a common motif in medicinal chemistry. Its incorporation into bioactive compounds is often accompanied by unwanted property modulation, such as increased lipophilicity and decreased metabolic stability. Several alternative substituents are available for the drug discovery process. Herein, physicochemical data of two series of drug analogues of bosentan and vercirnon are documented as part of a comparative study of tert-butyl, pentafluorosulfanyl, trifluoromethyl, bicyclo[1.1.1]pentanyl, and cyclopropyl-trifluoromethyl substituents.

The alternative scene: Given how common the tert-butyl group is in medicinal chemistry, we set out to explore some isosteric alternatives in hopes of identifying motifs that circumvent the common drawbacks imparted by tert-butyl groups, such as increased lipophilicity and lower metabolic stability.
Inhibition of Neuroinflammation by Synthetic Androstene Derivatives Incorporating Amino Acid Methyl Esters on Activated BV-2 Microglia
MChemSteroids
Abstract
Androstene derivatives incorporating amino acid methyl esters were prepared, and their anti-inflammatory effects were evaluated in lipopolysaccharide (LPS)-activated BV-2 microglial cells. Several compounds exhibited dose-dependent inhibition. The most active compound, methyl ((3S,10R,13S)-3-hydroxy-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthrene-17-carbonyl)-L-phenylalaninate (10) significantly suppressed LPS-induced expression of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α). Mechanistic studies revealed that compound 10 markedly inhibits phosphorylation of p38 mitogen-activated protein kinases (MAPKs) and subsequent transcription factor (NF-κB) and activator protein-1 (AP-1) activation. Furthermore, compound 10 decreased LPS-activated microglial neurotoxicity in a condition medium/HT-22 neuroblastoma co-culture model. Taken together, these results suggest 10 is a potential lead compound for the development of a novel therapeutic agent for neurodegenerative diseases.

Protecting the grey matter! Androstenes incorporating amino acid methyl esters were developed against neuroinflammation. Compound 10 markedly suppressed NO production and iNOS, COX-2, TNF-α and IL-6 expression in lipopolysaccharide-induced microglia. Compound 10 also inhibited phosphorylation of mitogen-activated protein kinases and subsequent activator protein-1 activation. These results suggest 10 as a candidate for development as an anti-neurodegenerative agent.
Stereoselective Synthesis of Highly Functionalized Dispirooxindoles through [3+2] Cycloaddition of Carbonyl Ylides with 3-Arylideneoxindoles
MChemspirooxindoles
Abstract
The three-component coupling (3CC) of α-diazoamide, aldehyde and 3-arylideneoxindole using 5 mol-% of Rh2(OAc)4 is described to afford a novel class of dispirooxindole derivatives with complete regio- and stereoselectivity. It is a first report on the synthesis of natural product inspired libraries of dispirooxindoles through a carbonyl ylide cycloaddition.

A highly stereoselective synthesis of furo-dispirooxindoles has been achieved by using 5 mol-% of Rh2(OAc)4 under mild conditions through 1,3-dipolar cycloaddition of 3-arylideneoxindoles, with carbonyl ylides that are generated from 3-diazooxindole and aldehyde.
Reversible Inhibitors of LSD1 as Therapeutic Agents in Acute Myeloid Leukemia: Clinical Significance and Progress to Date
MChemLSD1
Abstract
In the 10 years since the discovery of lysine-specific demethylase 1 (LSD1), this epigenetic eraser has emerged as an important target of interest in oncology. More specifically, research has demonstrated that it plays an essential role in the self-renewal of leukemic stem cells in acute myeloid leukemia (AML). This review will cover clinical aspects of AML, the role of epigenetics in the disease, and discuss the research that led to the first irreversible inhibitors of LSD1 entering clinical trials for the treatment of AML in 2014. We also review recent achievements and progress in the development of potent and selective reversible inhibitors of LSD1. These compounds differ in their mode of action from tranylcypromine derivatives and could facilitate novel biochemical studies to probe the pathways mediated by LSD1. In this review, we will critically evaluate the strengths and weaknesses of published series of reversible LSD1 inhibitors. Overall, while the development of reversible inhibitors to date has been less fruitful than that of irreversible inhibitors, there is still the possibility for their use to facilitate further research into the roles and functions of LSD1 and to expand the therapeutic applications of LSD1 inhibitors in the clinic.
Facile synthesis of spirooxindole-pyrazolines and spirobenzofuranone-pyrazolines and their fungicidal activity
MChemspirooxindole
DOI: 10.1039/C5OB00258C, Paper
A new and facile synthetic method is developed for biologically important spirooxindole-pyrazolines and spirobenzofuranone-pyrazolines.
The content of this RSS Feed (c) The Royal Society of Chemistry
Exploitation of the Ability of γ-Tocopherol to Facilitate Membrane Co-localization of Akt and PHLPP1 to Develop PHLPP1-Targeted Akt Inhibitors
MChemisosteric replacement
Synthesis of CDDO–Amino Acid–Nitric Oxide Donor Trihybrids as Potential Antitumor Agents against Both Drug-Sensitive and Drug-Resistant Colon Cancer
MChemSteroids
One-pot highly diastereoselective annulation to N-unprotected tetrasubstituted 2-pyrrolines
MChemGreen Chem
DOI: 10.1039/C4GC02191F, Communication
An effective one-pot sequential Michael addition/deprotection/cyclization/tautomerization approach to N-unprotected fully substituted trans-2-pyrrolines has been developed.
The content of this RSS Feed (c) The Royal Society of Chemistry
Sulfonamide formation from sodium sulfinates and amines or ammonia under metal-free conditions at ambient temperature
MChemOn water synthesis
DOI: 10.1039/C4GC02236J, Communication
A practical and highly efficient method for the construction of a variety of sulfonamides mediated by I2 was demonstrated. The reaction proceeds readily at room temperature using a variety of sodium sulfinates and amines or ammonia in water in a metal-, base-, ligand-, or additive-free protocol. Primary, secondary and tertiary sulfonamides were obtained in good to excellent yields with a broad range of substrate tolerability.
The content of this RSS Feed (c) The Royal Society of Chemistry
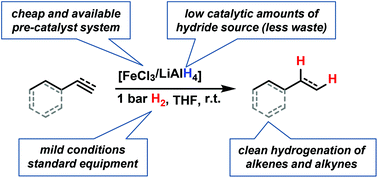
Iron-catalyzed olefin hydrogenation at 1 bar H2 with a FeCl3-LiAlH4 catalyst
DOI: 10.1039/C4GC02368D, Communication
The scope and mechanism of a practical protocol for the iron-catalyzed hydrogenation of alkenes and alkynes at 1 bar H2 pressure were studied.
The content of this RSS Feed (c) The Royal Society of Chemistry
Synthesis of sulfonamides via I2-mediated reaction of sodium sulfinates with amines in an aqueous medium at room temperature
MChemOn water synthesis
DOI: 10.1039/C4GC02115K, Communication
A convenient and green synthetic route has been developed to synthesize sulfonamides in an aqueous medium at room temperature, without the use of transition metal catalysts and oxidants.
The content of this RSS Feed (c) The Royal Society of Chemistry
"On water" catalysis: an expeditious approach for the synthesis of quaternary centered 3-hydroxy-3-(nitromethyl)indolin-2-one derivatives
MChemOn water synthesis
DOI: 10.1039/C4GC02203C, Communication
An efficient, eco-friendly "on water" method has been developed for the Henry reaction of isatins with nitromethane to afford 3-hydroxy-3-nitromethylindolin-2-ones. An enhancement in reaction rate was observed "on water" under mild and catalyst-free conditions.
The content of this RSS Feed (c) The Royal Society of Chemistry
Activity of 2-Aryl-2-(3-indolyl)acetohydroxamates against Drug-Resistant Cancer Cells
MChemMDR; Novel Scaffold
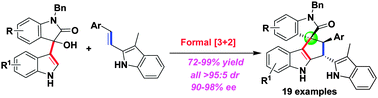
Highly diastereo- and enantioselective construction of a spiro[cyclopenta[b]indole-1,3[prime or minute]-oxindole] scaffold via catalytic asymmetric formal [3+2] cycloadditions
MChemoxindole
DOI: 10.1039/C4CC07246D, Communication
An organocatalytic asymmetric formal [3+2] cycloaddition of isatin-derived 3-indolylmethanol with 3-methyl-2-vinylindole has been established, leading to highly stereoselective construction of a spiro[cyclopenta[b]indole-1,3[prime or minute]-oxindole] scaffold.
The content of this RSS Feed (c) The Royal Society of Chemistry
N-heterocyclic carbene-catalyzed [4 + 2] cyclization of 2-bromo-2-enal with 3-alkylenyloxindoles: efficient assembly of spirocarbocyclic oxindole
MChemoxindole
DOI: 10.1039/C4OB01706D, Paper
A NHC-catalyzed [4 + 2] cyclization of 2-bromo-2-enal bearing [gamma]-H with 3-alkylenyloxindoles under mild reaction conditions gives spirocarbocyclic oxindoles containing one quaternary carbon with high diastereoselectivies.
The content of this RSS Feed (c) The Royal Society of Chemistry
Correction to p-Toluenesulfonic Acid Mediated 1,3-Dipolar Cycloaddition of Nitroolefins with NaN3 for Synthesis of 4-Aryl-NH-1,2,3-triazoles
MChemTriazole