Understanding the practical limitations of chemical reactions is critically important for efficiently planning the synthesis of compounds in pharmaceutical, agrochemical, and specialty chemical research and development. However, literature reports of the scope of new reactions are often cursory and biased toward successful results, severely limiting the ability to predict reaction outcomes for untested substrates. We herein illustrate strategies for carrying out large-scale surveys of chemical reactivity by using a material-sparing nanomole-scale automated synthesis platform with greatly expanded synthetic scope combined with ultrahigh-throughput matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry (MALDI-TOF MS).
chgra27
Shared posts
Mapping the dark space of chemical reactions with extended nanomole synthesis and MALDI-TOF MS
Synergy between nanomaterials and volatile organic compounds for non-invasive medical evaluation
DOI: 10.1039/C8CS00317C, Review Article
This review provides an insight into nanomaterial-based sensors for disease diagnostics via the detection of volatile organic compounds (VOCs).
The content of this RSS Feed (c) The Royal Society of Chemistry
Transition-Metal-Catalyzed Cross-Couplings through Carbene Migratory Insertion
Discovery and Total Synthesis of Natural Cystobactamid Derivatives with Superior Activity against Gram-Negative Pathogens
Abstract
Antibiotic discovery and development is challenging as chemical scaffolds of synthetic origin often lack the required pharmaceutical properties, and the discovery of novel ones from natural sources is tedious. Herein, we report the discovery of new cystobactamids with a significantly improved antibacterial profile in a detailed screening of myxobacterial producer strains. Some of these new derivatives display antibacterial activities in the low-μg mL−1 range against Gram-negative pathogens, including clinical isolates of Klebsiella oxytoca, Pseudomonas aeruginosa, and fluoroquinolone-resistant Enterobacteriaceae, which were not observed for previously reported cystobactamids. Our findings provide structure–activity relationships and show how pathogen resistance can be overcome by natural scaffold diversity. The most promising derivative 861-2 was prepared by total synthesis, enabling further chemical optimization of this privileged scaffold.

From Nature's toolbox: A concise study with myxobacterial producer strains yielded novel cystobactamid derivatives that display superior activity against Gram-negative pathogens. The most promising derivative was obtained by total synthesis, and will serve as a starting point for the further optimization of these gyrase inhibitors and their development as antibacterial drugs. SAR=structure–activity relationship.
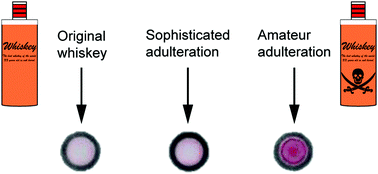
A paper-based colorimetric spot test for the identification of adulterated whiskeys
DOI: 10.1039/C7CC02271A, Communication
Seized adulterated whiskey samples were identified via a cheap, simple, colorimetric paper-analytic device.
The content of this RSS Feed (c) The Royal Society of Chemistry
Multivalent Siderophore–DOTAM Conjugates as Theranostics for Imaging and Treatment of Bacterial Infections
Abstract
There is a strong need to better diagnose infections at deep body sites through noninvasive molecular imaging methods. Herein, we describe the synthesis and characterization of probes based on siderophore conjugates with catechol moieties and a central DOTAM scaffold. The probes can accommodate a metal ion as well as an antibiotic moiety and are therefore suited for theranostic purposes. The translocation of the conjugates across the outer and inner cell membranes of E. coli was confirmed by growth recovery experiments with enterobactin-deficient strains, by the antibacterial activity of ampicillin conjugates, and by confocal imaging using a fluorogen-activating protein–malachite green system adapted to E. coli. The suitability of the probes for in vivo imaging was demonstrated with a Cy5.5 conjugate in mice infected with P. aeruginosa.

Trojan horses: A siderophore motif was coupled to functional imaging and/or antibiotic moieties via a DOTAM scaffold. The conjugates are internalized into Gram-positive as well as Gram-negative pathogenic bacteria and can be used for multiple theranostic applications, such as the in vivo imaging of infections.
[11C]Cyanation of arylboronic acids in aqueous solutions
DOI: 10.1039/C7CC02886E, Communication
A copper-mediated 11C-cyanation method employing arylboronic acids and [11C]HCN in aqueous solutions has been developed to provide a wide range of aromatic 11C-nitriles.
The content of this RSS Feed (c) The Royal Society of Chemistry
Modern Aspects of Isotopic Labellings in Terpene Biosynthesis
In this review article recent isotopic labelling experiments are presented that served in the elucidation of the complex mechanisms of terpene biosynthesis. The article is structured by first presenting the recent advances in the biosynthesis of terpene monomers, followed by sections on monoterpenes, sesquiterpenes and diterpenes. This overview covers a personal selection of eminent examples from the accumulated literature since 2010.

This review summarises the results of recent mechanistic investigations on enzymes involved in the biosynthesis of terpene precursors and on terpene cyclases by isotopic labelling experiments. The accumulated knowledge from these experiments is discussed against the background of insights obtained by quantum chemical calculations.
Enzyme-Inspired Chiral Secondary-Phosphine-Oxide Ligand with Dual Noncovalent Interactions for Asymmetric Hydrogenation
Abstract
Inspired by the unique character of enzymes, we developed novel chiral SPO (secondary-phosphine-oxide) ligand (SPO-Wudaphos) which can enter into both ion pair and H-bond noncovalent interactions. The novel chiral SPO-Wudaphos exhibited excellent results in the asymmetric hydrogenation of α-methylene-γ-keto carboxylic acids, affording the chiral γ-keto acids with up to over 99 % ee. A series of control experiments and DFT calculations were conducted to illustrate the critical roles of both the ion pair and H-bond noncovalent interactions.

Two different interactions: Just like enzymes which can enter into multiple cooperative noncovalent interactions with substrates, the chiral secondary-phosphine-oxide (SPO) Wudaphos ligand can engage in ion pair and H-bonding interactions. The chiral SPO-Wudaphos ligand gave excellent results in the rhodium-catalyzed asymmetric hydrogenation of α-methylene-γ-keto carboxylic acids.
Catalytic Asymmetric Bromocyclization of Polyenes
Regioselective 5-endo-dig Electrophilic Iodocyclization of Enediynes: A Convenient Route to Iodo-substituted Indenes and Cyclopenta-Fused Arenes
Abstract
An efficient iodine-mediated regioselective tandem approach for the synthesis of symmetric and asymmetric iodo-substituted indenes and stereoselective cyclopenta [b]pyridine/thiophenes from easily accessible enediynes that proceeds by in situ formation of an iodonium intermediate followed by a regioselective 5-endo-dig cyclization has been described. The intramolecular electrophilic iodocyclization was selectively triggered by a distribution of electronic density along the alkyne bond. Subsequently, the iodo-substituted indenes were diversified by employing palladium-catalyzed cross-coupling reactions and the coupled products were further confirmed by X-ray crystallographic studies.

An efficient iodine-mediated regioselective tandem approach for the synthesis of symmetric and asymmetric iodo-substituted indenes and stereoselective cyclopenta [b]pyridine/thiophenes using easily accessible enediynes has been described. The intramolecular electrophilic iodocyclization was selectively triggered by a distribution of electronic density along the alkyne bond. Subsequently, the iodo-substituted indenes were diversified by employing palladium-catalyzed cross-coupling reactions.
Chemistry and Biology of HPAs: A Family of Ceramide Trafficking Inhibitors
Abstract
In 2001, two years before the disclosure of the CERT-associated Cer transfer machinery, N-(3-hydroxy-1-hydroxymethyl-3-phenylpropyl)alkanamides (HPAs) were described as the first, and to date unique, family of intracellular Cer trafficking inhibitors. The dodecanamide derivative, HPA-12, turned out to be a benchmark as a cellular inhibitor of CERT-mediated de novo sphingomyelin biosynthesis. In only 15 years after its first disclosure, this compound has prompted a growing number of biological and chemical studies. Its initial chemical development closely paralleled the study of the CERT protein. It was only after its structural revision in 2011 that HPA-12 received broad attention from the synthetic chemistry community, leading to novel analogues with enhanced protein binding. This Minireview aims at presenting an exhaustive report of the syntheses of HPA-12 and analogues. Biological activities of this CERT inhibitor and structure–activity relationships are also presented to afford a comprehensive overview of the chemistry and biology of the HPA series.

In 2001, two years before the disclosure of the CERT-associated Cer transfer machinery, N-(3-hydroxy-1-hydroxymethyl-3-phenylpropyl)alkanamides (HPAs) were described as the first family of intracellular Cer trafficking inhibitors. The dodecanamide derivative, HPA-12, turned out to be a benchmark as a cellular inhibitor of CERT-mediated de novo sphingomyelin biosynthesis. This Minireview aims to present an exhaustive report of the chemistry and biology of the HPA series (see figure).
Iron-catalyzed Cross-Coupling of Propargyl Carboxylates and Grignard Reagents: Synthesis of Substituted Allenes
chgra27Enorme l'ACIE...
Abstract
Presented herein is a mild, facile, and efficient iron-catalyzed synthesis of substituted allenes from propargyl carboxylates and Grignard reagents. Only 1–5 mol % of the inexpensive and environmentally benign [Fe(acac)3] at −20 °C was sufficient to afford a broad range of substituted allenes in excellent yields. The method tolerates a variety of functional groups.

Iron clad: The title reaction is effective for the synthesis of substituted allenes using a low catalyst loading at −20 °C. The mild reaction conditions tolerated not only terminal acetylenes but also functional groups such as acetal, silyl ether, and ethyl carboxylate. A high degree of chirality-transfer was observed and the reaction conditions are compatible with radical probes.
Synthesis of Highly Functionalized 4-Aminoquinolines
Abstract
A diverse set of highly substituted 4-aminoquinolines was synthesized from ynamides, triflic anhydride, 2-chloropyridine, and readily accessible amides in a mild one-step procedure.

Diverse products: Electrophilically activated amides easily react with sulfonyl ynamides to yield a diverse range of substituted 4-aminoquinolines. The ynamides can be easily modified by Sonogashira processes or generated from dichloroenamide precursors. Any substituent can thus be introduced at the C3 position of the quinoline. 2-ClPy=2-chloropyridine, Tf2O=triflic anhydride.
Otter Does Not Want to Get Up, Okay?
chgra27Il y a une loutre dans notre sdb?
This is otter Ryer being woken up by his keeper at the Aquarium of the Bay in San Francisco!
Un nouveau projet de distillerie sur Islay
chgra27ils sont bien mes asaps hein?
L’embouteilleur indépendant Hunter Laing vient de dévoiler un projet sur l’île la plus cotée de la planète whisky. Il deviendrait ainsi le sixième négociant écossais à se lancer dans la production de scotch.
S’agira-t-il de la neuvième ou de la dixième distillerie d’Islay ? Les paris sont ouverts, alors que Jean Donnay, le patron de la distillerie bretonne Glann Ar Mor, a confirmé, mi-novembre, que son projet de distillerie sur l’île, Gartbreck, dévoilé il y a bientôt deux ans, entrerait dans une phase de construction au printemps 2016, pour une mise en service un an plus tard, au printemps ou à l’été 2017.
De son côté, l’embouteilleur indépendant Hunter Laing a annoncé, en fin de semaine dernière, la construction d’une nouvelle distillerie à Ardnahoe, à mi-chemin entre celles de Caol Ila et de Bunnahabhain, sur la côte nord-est de l’île, pour un montant de 8 millions de livres sterling (environ 10,5 millions d’euros). Le dossier est en cours d’examen par les autorités administratives et les travaux pourraient commencer dès mai, pour une mise en service fin 2017. La distillerie devrait disposer d’une capacité de production de 500.000 litres d’alcool pur par an (lpa), même si seulement 200.000 lpa devraient être distillés la première année de fonctionnement.
Une famille historique du scotch
La société Hunter Laing, fondée en 2013, est récente, mais la famille Laing travaille dans le whisky depuis des décennies. En désaccord, les frères Laing, Stewart et Fred, se sont en effet partagés l’affaire familiale – Fred conservant le nom de la société historique, Douglas Laing & Co, avec les marques Old Particular, XOP, Provenance et Director’s Cut, entre autres. De son côté, Stewart a fondé la société Hunter Laing, qu’il dirige avec ses fils Andrew et Scott, qui comprend la marque patrimoniale Old Malt Cask, ainsi qu’Old & Rare ou encore Douglas of Drumlanrig.
Ces dernières années, les grands embouteilleurs indépendants écossais ont pris l’habitude de se lancer dans la distillation, en rachetant des distilleries en service ou à l’arrêt, et même en en construisant de nouvelles. L’objectif : profiter de l’engouement actuel pour les single malts, bien sûr, mais aussi, sans doute, diversifier leurs activités afin de se mettre à l’abri d’éventuels problèmes d’approvisionnement à moyen terme sur le marché du négoce. Depuis quelques années, les distilleries conservent en effet leur production pour alimenter leurs marques officielles de single malts ou de blends.
Dans ce domaine, c’est Gordon & MacPhail qui a ouvert la voie dès 1993, avec le rachat de Benromach (Speyside) – remise en service en 1998. Avant d’être imité par Signatory Vintage avec Edradour (Highlands) en 2002, puis par Berry Bros & Rudd avec Glenrothes (Highlands) en 2010. Plus récemment les embouteilleurs Adelphi et Wemyss Malts ont emprunté la même voie, avec les constructions d’Ardnamurchan (Highlands) et Kingsbarns (Lowlands), entrées en production en 2014. Autant d’initiatives réussies, dont les amateurs attendent maintenant les premiers single malts.
Chiral N,N′-Dioxide–Scandium(III)-Catalyzed Asymmetric Dearomatization of 2-Naphthols through an Amination Reaction
chgra27c'est peut être pour ça qu'on s'est fait bouler de chem eur j...
Abstract
A catalytic asymmetric dearomatization of 2-naphthols with azodicarboxylates has been accomplished by using a N,N′-dioxide-scandium(III) complex as a chiral catalyst. A number of optically active β-naphthalenone compounds with a nitrogen-containing quaternary carbon stereocenter were obtained in up to 99 % yield and up to 99 % ee under mild reaction conditions. The reaction could be scaled up to a gram-scale with the yield and ee maintained. Based on these experiments and on previous reports, a possible transition state was proposed.

Animate you aminations! A catalytic asymmetric dearomatization of 2-naphthols with azodicarboxylates has been accomplished by using a N,N′-dioxide–scandium(III) complex as a chiral catalyst (see scheme). A number of optically active β-naphthalenone compounds with a nitrogen-containing quaternary carbon stereocenter were obtained under mild reaction conditions.
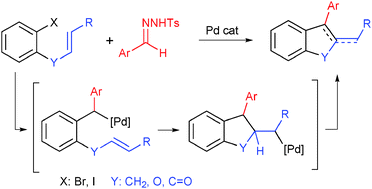
The Pd-catalyzed synthesis of benzofused carbo- and heterocycles through carbene migratory insertion/carbopalladation cascades with tosylhydrazones
DOI: 10.1039/C5CC06348E, Communication
Indenes and benzofurans are synthesized from tosylhydrazones and o-substituted aryliodides through a Pd-catalyzed carbene migratory insertion/intramolecular carbopalladation cascade reaction.
The content of this RSS Feed (c) The Royal Society of Chemistry
White Light Emission from an Integrated Upconversion Nanostructure: Toward Multicolor Displays Modulated by Laser Power
Abstract
The white backlight in displays is generated by optimizing the proportions of individual emitters with different wavelengths by variations in materials composition, phase, and structure. Color pixels usually result from the separation of white light or the excitation with multiwavelength or multipulse sources. However, it is a challenge to develop a material that comprises a single structure and emits over the full visible spectrum, but where the emission wavelengths can be controlled by a simple excitation source. Herein, we report an upconversion nanostructure that incorporates several lanthanide ions in the same core@shell@shell structure. The combination of multiple narrow spectral bands results in the emission of white light. The emission colors can be tuned by changing the excitation power density, which manipulates the photon transfer pathways. Applications such as flat-panel displays and imaging have been demonstrated.

Seeing the light: An upconversion nanostructure comprises several lanthanide ions integrated in a single system. The balance of numerous narrow emission bands covering the full visible spectrum results in white-light emission. The emission colors can be determined by changing the excitation power density (see picture), which manipulates the photon transfer pathways to bring potential applications such as multicolor displays or imaging.
First Total Synthesis of Pandamarine
Photo-induced Metal-Catalyst-Free Aromatic Finkelstein Reaction
Chiral Phosphoric Acid Catalyzed Asymmetric Synthesis of 2-Substituted 2,3-Dihydro-4-quinolones by a Protecting-Group-Free Approach
Vinylic MIDA Boronates: New Building Blocks for the Synthesis of Aza-Heterocycles
Abstract
A two-step synthesis of structurally diverse pyrrole-containing bicyclic systems is reported. ortho-Nitro-haloarenes coupled with vinylic N-methyliminodiacetic acid (MIDA) boronates generate ortho-vinyl-nitroarenes, which undergo a “metal-free” nitrene insertion, resulting in a new pyrrole ring. This novel synthetic approach has a wide substrate tolerance and it is applicable in the preparation of more complex “drug-like” molecules. Interestingly, an ortho-nitro-allylarene derivative furnished a cyclic β-aminophosphonate motif.

Old dog, new tricks: A two-step synthesis of structurally diverse pyrrole-containing bicyclic systems is reported. ortho-Nitro-haloarenes coupled with vinylic N-methyliminodiacetic acid (MIDA) boronates generate ortho-vinyl-nitroarenes, which undergo a “metal-free” nitrene insertion, resulting in a new pyrrole ring. This approach has a wide substrate tolerance and it is applicable in the preparation of more complex “drug-like” molecules. Interestingly, an ortho-nitro-allylarene derivative furnished a cyclic β-aminophosphonate motif.