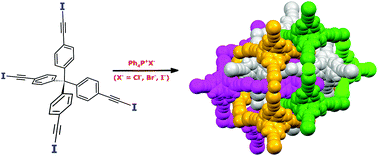Shared posts
Interlocked Supramolecular Polymers Created by Combination of Halogen- and Hydrogen-Bonding Interactions through Anion-Template Self-Assembly
Selective Anion Extraction and Recovery Using a FeII4L4 Cage
Abstract
Selective anion extraction is useful for the recovery and purification of valuable chemicals, and in the removal of pollutants from the environment. Here we report that FeII4L4 cage 1 is able to extract an equimolar amount of ReO4−, a high-value anion and a nonradioactive surrogate of TcO4−, from water into nitromethane. Importantly, the extraction was efficiently performed even in the presence of 10 other common anions in water, highlighting the high selectivity of 1 for ReO4−. The extracted guest could be released into water as the cage disassembled in ethyl acetate, and then 1 could be recycled by switching the solvent to acetonitrile. The versatile solubility of the cage also enabled complete extraction of ReO4− (as the tetrabutylammonium salt) from an organic phase into water by using the sulfate salt of 1 as the extractant.

A FeII4L4 coordination cage enabled complete extraction of ReO4−, a nonradioactive surrogate of TcO4−, from water into an organic phase. In the presence of 10 other anions, 97 % of ReO4− was selectively removed. The extracted ReO4− could be released and the cage extractant recycled by a solvent-switching strategy. Rendering the cage water-soluble also allowed complete extraction of ReO4− from an organic phase into water.
Anion-Mediated Photophysical Behavior in a C60 Fullerene [3]Rotaxane Shuttle
Synthesis of partially and fully fused polyaromatics by annulative chlorophenylene dimerization
Since the discovery by Ullmann and Bielecki in 1901, reductive dimerization (or homocoupling) of aryl halides has been extensively exploited for the generation of a range of biaryl-based functional molecules. In contrast to the single-point connection in these products, edge-sharing fused aromatic systems have not generally been accessible from simple aryl halides via annulation cascades. Here we report a single-step synthesis of fused aromatics with a triphenylene core by the palladium-catalyzed annulative dimerization of structurally and functionally diverse chlorophenylenes through double carbon-hydrogen bond activation. The partially fused polyaromatics can be transformed into fully fused, small graphene nanoribbons, which are otherwise difficult to synthesize. This simple, yet powerful, method allows access to functional -systems of interest in optoelectronics research.
Redox-Active Ligand Assisted Multielectron Catalysis: A Case of CoIII Complex as Water Oxidation Catalyst
Rewiring Chemical Networks Based on Dynamic Dithioacetal and Disulfide Bonds
Abstract
The control of the connectivity between nodes of synthetic networks is still largely unexplored. To address this point we take advantage of a simple dynamic chemical system with two exchange levels that are mutually connected and can be activated simultaneously or sequentially. Dithioacetals and disulfides can be exchanged simultaneously under UV light in the presence of a sensitizer. Crossover reactions between both exchange processes produce a fully connected chemical network. On the other hand, the use of acid, base or UV light connects different nodes allowing network rewiring.

Network rewiring: Molecular networks with markedly different structures can be rewired through the simultaneous or sequential activation of dithioacetal and disulfide exchanges.
Fuel-Selective Transient Activation of Nanosystems for Signal Generation
Matching fuel for action: The transient activation of function by using chemical fuels is common in nature, but much less in synthetic systems. In a newly developed system transient signal generation is only observed when the chemical fuel matches the recognition site in monolayer-protected gold nanoparticles.
[Communication]
Flavio della Sala, Subhabrata Maiti, Andrea Bonanni, Paolo Scrimin, Leonard J. Prins
Angew. Chem. Int. Ed., January 12, 2018, https://doi.org/10.1002/anie.201711964 Read article
Peripheral Templation-Modulated Interconversion between an A4L6 Tetrahedral Anion Cage and A2L3 Triple Helicate with Guest Capture/Release
In and out: An unusual peripherally templated A4L6 tetrahedral cage is reported, which can be transformed into an A2L3 helicate in response to different stimuli (template, concentration, or solvent), with accompanying guest capture and release (see picture; A=anion, L=ligand).
[Communication]
Xuemin Bai, Chuandong Jia, Yanxia Zhao, Dong Yang, Shi-Cheng Wang, Anyang Li, Yi-Tsu Chan, Yao-Yu Wang, Xiao-Juan Yang, Biao Wu
Angew. Chem. Int. Ed., January 08, 2018, https://doi.org/10.1002/anie.201712080 Read article
Reprograming the Replisome of a Semisynthetic Organism for the Expansion of the Genetic Alphabet
Mechanically robust, readily repairable polymers via tailored noncovalent cross-linking
Expanding the range of healable materials is an important challenge for sustainable societies. Noncrystalline, high-molecular-weight polymers generally form mechanically robust materials, which, however, are difficult to repair once they are fractured. This is because their polymer chains are heavily entangled and diffuse too sluggishly to unite fractured surfaces within reasonable time scales. Here we report that low-molecular-weight polymers, when cross-linked by dense hydrogen bonds, yield mechanically robust yet readily repairable materials, despite their extremely slow diffusion dynamics. A key was to use thiourea, which anomalously forms a zigzag hydrogen-bonded array that does not induce unfavorable crystallization. Another key was to incorporate a structural element for activating the exchange of hydrogen-bonded pairs, which enables the fractured portions to rejoin readily upon compression.
Surface-Confined Dynamic Covalent System Driven by Olefin Metathesis
Abstract
Understanding how the constitutional dynamics of a dynamic combinatorial library (DCL) adapts to surfaces (compared to bulk solution) is of fundamental importance to the design of adaptive materials. Submolecular resolved scanning tunneling microscopy (STM) can provide detailed insights into olefin metathesis at the interface. Analysis of the distribution of products has revealed the important role of environmental pressure, reaction temperature, and substituent effects in surface-confined olefin metathesis. We also report an unprecedented preferred deposition and assembly of linear polymers, and some specific oligomers, on the surface that are hard to obtain otherwise.

Surface-confined olefin metathesis was studied by scanning tunneling microscopy to determine the effects of substituents, reaction temperature, and pressure. Surface confinement significantly enhances the formation of linear polymers and allows selective synthesis of specific oligomers that are difficult to obtain by solution-phase synthesis. DCC=Dynamic covalent chemistry.
Fuel-Selective Transient Activation of Nanosystems for Signal Generation
Abstract
The transient activation of function using chemical fuels is common in nature, but much less in synthetic systems. Progress towards the development of systems with a complexity similar to that of natural ones requires chemical fuel selectivity. Here, we show that a self-assembled nanosystem, composed of monolayer-protected gold nanoparticles and a fluorogenic peptide, is activated for transient signal generation only in case the chemical fuel matches the recognition site present at the nanoparticle surface. A modification of the recognition site in the nanosystem completely changes the chemical fuel selectivity. When two nanosystems are simultaneously present, the selectivity expressed by the system depends on the concentration of nucleotide added.

Matching fuel for action: The transient activation of function by using chemical fuels is common in nature, but much less in synthetic systems. In a newly developed system transient signal generation is only observed when the chemical fuel matches the recognition site in monolayer-protected gold nanoparticles.
First use of a divalent lanthanide for visible-light-promoted photoredox catalysis
DOI: 10.1039/C7SC02479G, Edge Article
 Open Access
Open Access   This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.
  This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.
Divalent europium is used catalytically in visible-light-promoted photoredox bond-forming reactions.
The content of this RSS Feed (c) The Royal Society of Chemistry
Synthesis of imidazole-activated ribonucleotides using cyanogen chloride
DOI: 10.1039/C7CC08489G, Communication
Just add cyanide and bleach! Aqueous cyanide anion, hypochlorous acid, imidazole and ribonucleoside 5[prime or minute]-monophosphate furnish in one-pot the chemically activated 5[prime or minute]-phosphorimidazolides.
The content of this RSS Feed (c) The Royal Society of Chemistry
Diamondoid architectures from halogen-bonded halides
DOI: 10.1039/C7CC08839F, Communication
Halide ions and tetraiodoethynyl-featured tetraphenylmethane are successfully assembled into robust diamond-like networks in the presence of tetraphenylphosphonium cations.
The content of this RSS Feed (c) The Royal Society of Chemistry
Supramolecular Chemistry of Anionic Dimers, Trimers, Tetramers, and Clusters
Poly[n]catenanes: Synthesis of molecular interlocked chains
As the macromolecular version of mechanically interlocked molecules, mechanically interlocked polymers are promising candidates for the creation of sophisticated molecular machines and smart soft materials. Poly[n]catenanes, where the molecular chains consist solely of interlocked macrocycles, contain one of the highest concentrations of topological bonds. We report, herein, a synthetic approach toward this distinctive polymer architecture in high yield (~75%) via efficient ring closing of rationally designed metallosupramolecular polymers. Light-scattering, mass spectrometric, and nuclear magnetic resonance characterization of fractionated samples support assignment of the high–molar mass product (number-average molar mass ~21.4 kilograms per mole) to a mixture of linear poly[7–26]catenanes, branched poly[13–130]catenanes, and cyclic poly[4–7]catenanes. Increased hydrodynamic radius (in solution) and glass transition temperature (in bulk materials) were observed upon metallation with Zn2+.
Enzyme-free nucleic acid dynamical systems
Chemistries exhibiting complex dynamics—from inorganic oscillators to gene regulatory networks—have been long known but either cannot be reprogrammed at will or rely on the sophisticated enzyme chemistry underlying the central dogma. Can simpler molecular mechanisms, designed from scratch, exhibit the same range of behaviors? Abstract chemical reaction networks have been proposed as a programming language for complex dynamics, along with their systematic implementation using short synthetic DNA molecules. We developed this technology for dynamical systems by identifying critical design principles and codifying them into a compiler automating the design process. Using this approach, we built an oscillator containing only DNA components, establishing that Watson-Crick base-pairing interactions alone suffice for complex chemical dynamics and that autonomous molecular systems can be designed via molecular programming languages.
Dynamic Covalent Chemistry within Biphenyl Scaffolds: Reversible Covalent Bonding, Control of Selectivity, and Chirality Sensing with a Single System
Abstract
Axial chirality is a prevalent and important phenomenon in chemistry. Herein we report a combination of dynamic covalent chemistry and axial chirality for the development of a versatile platform for the binding and chirality sensing of multiple classes of mononucleophiles. An equilibrium between an open aldehyde and its cyclic hemiaminal within biphenyl derivatives enabled the dynamic incorporation of a broad range of alcohols, thiols, primary amines, and secondary amines with high efficiency. Selectivity toward different classes of nucleophiles was also achieved by regulating the distinct reactivity of the system with external stimuli. Through induced helicity as a result of central-to-axial chirality transfer, the handedness and ee values of chiral monoalcohol and monoamine analytes were reported by circular dichroism. The strategies introduced herein should find application in many contexts, including assembly, sensing, and labeling.

Talking sense: A convergence of the concepts of dynamic covalent chemistry and axial chirality enabled the reversible incorporation and chirality sensing of multiple classes of mononucleophiles (see picture). Selectivity toward different classes of nucleophiles could also be induced by regulating the distinct reactivity of the system with external stimuli.
Gram-Scale Syntheses and Conductivities of [10]Cycloparaphenylene and Its Tetraalkoxy Derivatives
Expansion of the Genetic Alphabet: A Chemist’s Approach to Synthetic Biology
Out-of-Equilibrium Aggregates and Coatings during Seeded Growth of Metallic Nanoparticles
Photoredox-catalyzed deuteration and tritiation of pharmaceutical compounds
Deuterium- and tritium-labeled pharmaceutical compounds are pivotal diagnostic tools in drug discovery research, providing vital information about the biological fate of drugs and drug metabolites. Herein we demonstrate that a photoredox-mediated hydrogen atom transfer protocol can efficiently and selectively install deuterium (D) and tritium (T) at α-amino sp3 carbon-hydrogen bonds in a single step, using isotopically labeled water (D2O or T2O) as the source of hydrogen isotope. In this context, we also report a convenient synthesis of T2O from T2, providing access to high-specific-activity T2O. This protocol has been successfully applied to the high incorporation of deuterium and tritium in 18 drug molecules, which meet the requirements for use in ligand-binding assays and absorption, distribution, metabolism, and excretion studies.
Binding of Hydrophobic Guests in a Coordination Cage Cavity is Driven by Liberation of “High-Energy” Water
Abstract
Invited for the cover of this issue is the group of Michael D. Ward at the Universities of Warwick and Sheffield. The image depicts the ‘hydrophobic effect’ that is responsible for guest binding in the host cavity. Read the full text of the article at 10.1002/chem.201704163.

“Using a combination of X-ray crystallography and NMR studies, we have demonstrated the extensive host/guest properties of our cubic cage.” Read more about the story behind the cover in the Cover Profile and about the research itself on page ▪▪ ff. (DOI: 10.1002/chem.201704163).
Exploiting the Strong Hydrogen Bond Donor Properties of a Borinic Acid Functionality for Fluoride Anion Recognition
Abstract
Borinic acids have typically not been considered as hydrogen bond donor groups in molecular recognition. Described herein is a bifunctional borane/borinic acid derivative (2) in which the two functionalities are connected by a 1,8-biphenylenediyl backbone. Anion binding studies reveal that 2 readily binds a fluoride anion by formation of a unique B−F⋅⋅⋅H−O−B hydrogen bond. This hydrogen bond is characterized by a short H-F distance of 1.79(3) Å and a large coupling constant (1JHF) of 57.2 Hz. The magnitude of this interaction, which has also been investigated computationally, augments the fluoride anion binding properties of 2, thus making it compatible with aqueous environments.

A Brønsted and Lewis handshake: The borinic acid functionality of a new diboron electrophilic host reaches across the binding pocket to engage a borane-bound fluoride anion in a strong hydrogen-bonding interaction. The resulting B−F⋅⋅⋅H−O−B interaction stabilizes the complex in aqueous solutions, thereby illustrating the role that borinic acids may play as hydrogen bond donor groups.
Resolving a Reactive Organometallic Intermediate from Dynamic Directing Group Systems by Selective C−H Activation
Abstract
Catalyst discovery from systems of potential precursors is a challenging endeavor. Herein, a new strategy applying dynamic chemistry to the identification of catalyst precursors from C−H activation of imines is proposed and evaluated. Using hydroacylation of imines as a model reaction, the selection of an organometallic reactive intermediate from a dynamic imine system, involving many potential directing group/metal entities, is demonstrated. The identity of the amplified reaction intermediate with the best directing group could be resolved in situ by ESI-MS, and coupling of the procedure to an iterative deconvolution protocol generated a system with high screening efficiency.

In the director′s chair: A new strategy, applying dynamic chemistry to the selection of catalyst precursors from C−H activation of imines is presented. Using hydroacylation as a model reaction, the strategy enabled identification of an organometallic reactive intermediate from a dynamic imine system, where the best directing group could be resolved in situ.
Self-sorted pore-formation in the construction of heteropore covalent organic frameworks based on orthogonal reactions
DOI: 10.1039/C7CC07808K, Communication
Covalent organic frameworks bearing two different kinds of micropores have been constructed based on the orthogonal formation of dynamic covalent bonds. The orthogonal reactions result in an unprecedented self-sorted pore-formation in the polymerization process.
To cite this article before page numbers are assigned, use the DOI form of citation above.
The content of this RSS Feed (c) The Royal Society of Chemistry
Supramolecular Chemistry: A Toolkit for Soft Functional Materials and Organic Particles
Perovskite solar cells with CuSCN hole extraction layers yield stabilized efficiencies greater than 20%
Perovskite solar cells (PSCs) with efficiencies greater than 20% have been realized only with expensive organic hole-transporting materials. We demonstrate PSCs that achieve stabilized efficiencies exceeding 20% with copper(I) thiocyanate (CuSCN) as the hole extraction layer. A fast solvent removal method enabled the creation of compact, highly conformal CuSCN layers that facilitate rapid carrier extraction and collection. The PSCs showed high thermal stability under long-term heating, although their operational stability was poor. This instability originated from potential-induced degradation of the CuSCN/Au contact. The addition of a conductive reduced graphene oxide spacer layer between CuSCN and gold allowed PSCs to retain >95% of their initial efficiency after aging at a maximum power point for 1000 hours under full solar intensity at 60°C. Under both continuous full-sun illumination and thermal stress, CuSCN-based devices surpassed the stability of spiro-OMeTAD–based PSCs.
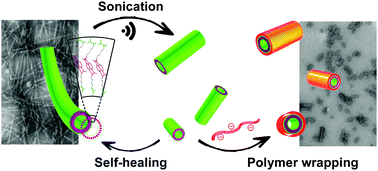
Controlling the length of self-assembled nanotubes by sonication followed by polymer wrapping
DOI: 10.1039/C7CC07418B, Communication
In this work, we report that sonication, followed by polymer-wrapping, is an effective strategy to reduce the length of self-assembled nanotubes and suspend their propensity to self-heal into their elongated precursors.
The content of this RSS Feed (c) The Royal Society of Chemistry