Shared posts
Nanocellulose Incorporated Liquid Crystal Elastomers as Soft Actuators
Selective Leaching and Recovery of Er, Gd, Sn, and In from Liquid Crystal Display Screen Waste by Sono-Leaching Assisted by Magnetic Separation
Dependence of the Core–Shell Structure on the Lipid Composition of Nanostructured Lipid Carriers: Implications for Drug Carrier Design
Enantiodifferentiating Photodimerization of a 2,6‐Disubstituted Anthracene Assisted by Supramolecular Double‐Helix Formation with Chiral Amines

A 2,6‐anthrylene‐linked bis(m‐terphenylcarboxylic acid) strand forms a one‐handed homo double helix induced by chiral amines, thereby producing the chiral anti‐photodimer with up to 98 % enantiomeric excess upon photoirradiation. The chirality of the anti‐photodimer can be readily controlled by the chirality of the chiral amines.
Abstract
A novel 2,6‐anthrylene‐linked bis(m‐terphenylcarboxylic acid) strand (1) self‐associates into a racemic double‐helix. In the presence of chiral mono‐ and diamines, either a right‐ or left‐handed double‐helix was predominantly induced by chiral amines sandwiched between the carboxylic acid strands with accompanying stacking of the two prochiral anthracene linker units in an enantiotopic face‐selective way, as revealed by circular dichroism and NMR spectral analyses. The photoirradiation of the optically active double helices complexed with chiral amines proceeded in a diastereo‐ (anti or syn) and enantiodifferentiating way to afford the chiral anti‐photodimer with up to 98 % enantiomeric excess when (R)‐phenylethylamine was used as a chiral double‐helix inducer. The resulting optically active anti‐photodimer can recognize the chirality of amines and diastereoselectively complex with chiral amines.
Alternative to the Popular Imidazolium Ionic Liquids: 1,2,4-Triazolium Ionic Liquids with Enhanced Thermal and Chemical Stability
Interfacial Engineering for the Synergistic Enhancement of Thermal Conductivity of Discotic Liquid Crystal Composites
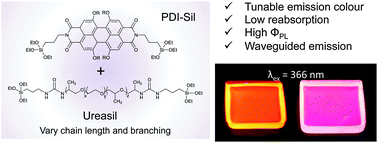
Targeted design leads to tunable photoluminescence from perylene dicarboxdiimide-poly(oxyalkylene)/siloxane hybrids for luminescent solar concentrators
DOI: 10.1039/C5TC03952E, Paper
The chain length and branching of the organic backbone of poly(oxyalkylene)/siloxane ureasils can be used to control the placement and orientation of a covalently-grafted perylene, leading to tunable photoluminescence.
The content of this RSS Feed (c) The Royal Society of Chemistry