07 Oct 16:56
by Jorge Bañuelos, Antonio Gómez-Infante, Ismael Valois-Escamilla, David Cruz-Cruz, Ruth Prieto-Montero, Iñigo Lopez-Arbeloa, Teresa Arbeloa, Eduardo Peña-Cabrera
Five new nonsymmetric BODIPY dyes based on the Biellmann methodology [NSBBs (nonsymmetrical Biellmann BODIPYs)] were prepared by condensation of pairs of different pyrroles with thiophosgene. Treatment of the resulting thioketones with MeI and BF3·Et2O/TEA yielded a wide range of products in a simple manner, with selective alkylation of each pyrrole of the indacene core and with controlled functionalization at the meso position. A comprehensive combined photophysical and computational study has been conducted to determine the impact of the alkylation pattern, as well as the role of the conformational freedom and electron coupling of 8-phenyl and 8-methylthio groups on the spectroscopic signatures of BODIPYs. The results and conclusions reached are compared with those for previously reported related analogues. We intend to provide key structural guidelines with regard to the factors that trigger the fluorescence responses and spectral shifts of multifunctional fluorophores of this family.

New nonsymmetric 8-methylthioBODIPYs featuring selective alkylation of the pyrrole systems were synthesized and their specific functionalization at the meso position was achieved. The combined photophysical and computational characterization of these dyes provides key structural guidelines for modulation of their spectral band positions, as well as their fluorescence responses.
01 Sep 19:36
Chem. Commun., 2016, 52,11563-11566
DOI: 10.1039/C6CC06344F, Communication
Natalia O. Didukh, Yuriy V. Zatsikha, Gregory T. Rohde, Tanner S. Blesener, Viktor P. Yakubovskyi, Yuriy P. Kovtun, Victor N. Nemykin
Diferrocene-containing meso-cyano-BODIPY (4) was prepared and characterized by spectroscopy, X-ray crystallography, and DFT calculations.
The content of this RSS Feed (c) The Royal Society of Chemistry

01 Sep 19:29
Chem. Sci., 2016, Advance Article
DOI: 10.1039/C6SC03288E, Edge Article

Open Access
Yingxia Wang, Hao Cui, Zhang-Wen Wei, Hai-Ping Wang, Li Zhang, Cheng-Yong Su
A robust iridium(III)-porphyrin metal-organic framework acts as a selective catalyst for carbenoid insertion into primary Si-H bonds with excellent effectivity.
To cite this article before page numbers are assigned, use the DOI form of citation above.
The content of this RSS Feed (c) The Royal Society of Chemistry

15 Aug 11:38
by Shawn Swavey, Michael Coladipietro, Aliah Burbayea, Jeanette A. Krause
A two-step synthetic route toward BODIPY dyes is accomplished by reaction of the readily available naphtho[1,2-c]pyrrole with various aldehydes followed by coordination of BF2. Synthesis of the dipyrromethene is achieved by simply heating the pyrrole and aldehyde at moderate temperatures for less than 30 min. In addition to the absence of solvent this reaction does not require an acid catalyst or an oxidizing agent to achieve the dipyrromethene. Investigation of various aldehydes by this method suggests that the choice of aldehyde plays a role in the formation of either symmetric or asymmetric BODIPYs. The dyes have exceptionally high molar absorptivities (> 100000 m–1 cm–1) in the far visible region of the electromagnetic spectrum with intense emission and high quantum efficiency.

A series of naphthyl-fused BODIPY dyes have been synthesized by a simple two-step process. These dyes display high molar absorptivities in the far visible region of the spectrum with emission quantum efficiencies at or near unity.
10 Aug 10:47

Looks like a job for a copper: The integration of Cu2+ with graphitic carbon nitride (g-C3N4) nanosheets greatly improved the efficiency of these photosensitizers for photodynamic therapy. The observed improvement in cytotoxicity was due to the enhanced light-triggered generation of reactive oxygen species in combination with the depletion of intracellular glutathione (GSH) levels (see picture).
[Communication]
Enguo Ju, Kai Dong, Zhaowei Chen, Zhen Liu, Chaoqun Liu, Yanyan Huang, Zhenzhen Wang, Fang Pu, Jinsong Ren, Xiaogang Qu
Angew. Chem. Int. Ed., August 09, 2016, DOI: 10.1002/anie.201605509. Read article
10 Aug 10:45
by Gamidi Rama Krishna, Ramesh Devarapalli, Garima Lal and C. Malla Reddy

Journal of the American Chemical Society
DOI: 10.1021/jacs.6b05118

10 Aug 10:40
by Shin-ichiro Kato, Satoshi Kuwako, Nobutaka Takahashi, Tomokazu Kijima and Yosuke Nakamura

The Journal of Organic Chemistry
DOI: 10.1021/acs.joc.6b01409

10 Jun 13:35
by Marcin Stępień, Elżbieta Gońka, Marika Żyła and Natasza Sprutta

Chemical Reviews
DOI: 10.1021/acs.chemrev.6b00076

10 Jun 13:13
by Alaina M. Forbes, G. Patrick Meier, Ebenezer Jones-Mensah, Jakob Magolan
A procedure for the synthesis of biaryl ketones by a palladium-catalyzed Suzuki–Miyaura cross-coupling reaction between phenyltrifluoroborates and benzoyl chlorides is described. Organotrifluoroborates are unique to other cross-coupling reagents as they have a high functional-group tolerance and are moisture-stable. Moderate to excellent yields were obtained for all substrates tested.

A procedure for the synthesis of biaryl ketones by a Pd-catalyzed Suzuki–Miyaura cross-coupling reaction between phenyltrifluoroborates and benzoyl chlorides with moderate to excellent yields is described. Organotrifluoroborates are unique to other cross-coupling reagents as they have a high functional-group tolerance and are moisture-stable.
10 Jun 11:51
by Tetsuo Okujima, Chie Ando, Saurabh Agrawal, Hiroki Matsumoto, Shigeki Mori, Keishi Ohara, Ichiro Hisaki, Takahiro Nakae, Masayoshi Takase, Hidemitsu Uno and Nagao Kobayashi

Journal of the American Chemical Society
DOI: 10.1021/jacs.6b04941

25 May 14:35

Polycyclic Aromatics F. Würthner et al. describe in their Communication on page 6390 ff. a palladium-catalyzed cascade process to synthesize an electron-poor C64 nanographene. Its planar geometry was revealed by single-crystal X-ray analysis.
[Frontispiece]
Sabine Seifert, Kazutaka Shoyama, David Schmidt, Frank Würthner
Angew. Chem. Int. Ed., May 19, 2016, DOI: 10.1002/anie.201682261. Read article
25 May 14:02

The fast and the luminous: A new type of highly luminescent cesium lead halide nanoplatelets show fast anion exchange. Fast anion exchange in the as-synthesized CsPb2Br5 nanoplatelets has also been demonstrated to extend their photoluminescence spectra to the entire visible spectral region of 410 nm–670 nm.
[Communication]
Kun-Hua Wang, Liang Wu, Lei Li, Hong-Bin Yao, Hai-Sheng Qian, Shu-Hong Yu
Angew. Chem. Int. Ed., May 23, 2016, DOI: 10.1002/anie.201602787. Read article
17 Apr 12:27
by Xu Liu, Tiantian Cong, Ping Liu and Peipei Sun

The Journal of Organic Chemistry
DOI: 10.1021/acs.joc.6b00097

15 Apr 13:01
by Eduardo Palao, Gonzalo Duran-Sampedro, Santiago de la Moya, Miriam Madrid, Carmen García-López, Antonia R. Agarrabeitia, Bram Verbelen, Wim Dehaen, Nöel Boens and María J. Ortiz

The Journal of Organic Chemistry
DOI: 10.1021/acs.joc.6b00350

10 Apr 09:16

Ten bonds in a single operation: The synthesis of an electron-poor nanographene with dicarboximide corners was accomplished by a palladium-catalyzed cascade process, forming ten C−C bonds in a single chemical operation. The planar geometry of this novel C64 nanographene was revealed by single-crystal X-ray analysis.
[Communication]
Sabine Seifert, Kazutaka Shoyama, David Schmidt, Frank Würthner
Angew. Chem. Int. Ed., April 05, 2016, DOI: 10.1002/anie.201601433. Read article
07 Apr 10:10
by Bayrammurad Saparov and David B. Mitzi

Chemical Reviews
DOI: 10.1021/acs.chemrev.5b00715

02 Apr 10:28
by Tim Dumslaff, Bo Yang, Ali Maghsoumi, Gangamallaiah Velpula, Kunal S. Mali, Chiara Castiglioni, Steven De Feyter, Matteo Tommasini, Akimitsu Narita, Xinliang Feng and Klaus Müllen

Journal of the American Chemical Society
DOI: 10.1021/jacs.6b01976

25 Mar 16:53

Bulk discount on hexaarylbenzenes (HABs): A rational and scalable synthesis of uncommon and highly functionalized HABs utilizes 4-nitroaniline as the starting material. This approach can potentially provide 18 novel HABs and 26 substitution geometries in total, which are not available or only difficult to obtain by standard techniques.
[Communication]
Dominik Lungerich, David Reger, Helen Hölzel, René Riedel, Max M. J. C. Martin, Frank Hampel, Norbert Jux
Angew. Chem. Int. Ed., March 24, 2016, DOI: 10.1002/anie.201600841. Read article
17 Mar 18:02
Chem. Sci., 2016, 7,4355-4363
DOI: 10.1039/C6SC00559D, Edge Article

Open Access
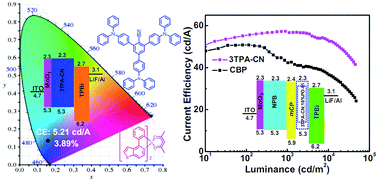
Xuejun Zhan, Zhongbin Wu, Yuxuan Lin, Yujun Xie, Qian Peng, Qianqian Li, Dongge Ma, Zhen Li
For the first time, AIEgens with good hole-transporting ability served as emitting layer or host with high performance.
The content of this RSS Feed (c) The Royal Society of Chemistry

16 Mar 16:21
Chem. Commun., 2016, 52,5402-5405
DOI: 10.1039/C6CC01048B, Communication
Weiqi Wang, Lei Wang, Zhensheng Li, Zhigang Xie
BODIPY-immobilized metal-organic frameworks for photodynamic therapy were developed through solvent-assisted ligand exchange.
The content of this RSS Feed (c) The Royal Society of Chemistry

16 Mar 16:19
Chem. Sci., 2016, 7,4301-4307
DOI: 10.1039/C5SC04783H, Edge Article

Open Access
Hongda Wang, Liangang Xiao, Lei Yan, Song Chen, Xunjin Zhu, Xiaobin Peng, Xingzhu Wang, Wai-Kwok Wong, Wai-Yeung Wong
Three A-D-A porphyrin-based small molecules are employed as donors in bulky heterojunction organic solar cells. Striking a delicate balance between solubility, morphology and device fabrication, leads to PCEs of up to 7.7%.
The content of this RSS Feed (c) The Royal Society of Chemistry

16 Mar 16:17
by Anna Chiara Sale, Adrian H. Murray, Dominik Prenzel, Frank Hampel, Lidia De Luca, Rik R. Tykwinski
Abstract
Diels–Alder cycloaddition reactions of 1,3,5-hexatriynes with tetraphenylcyclopentadienone have been performed, and mainly gave 1,2-diethynyl-3,4,5,6-tetraphenylbenzene derivatives as products. The Diels–Alder reactions were optimized under conventional heating and microwave irradiation conditions, and showed good selectivity towards the central carbon–carbon triple bond of the triyne. Empirical evidence suggests that the selectivity of the reaction is governed predominantly by the steric demands of the end-groups of the triyne. Several of the Diels–Alder products could be further elaborated by a sequence of desilylation and oxidative homocoupling to provide dimeric products. The homocoupling reaction was optimized by Pd catalysis. The X-ray crystallographic analysis of two derivatives is reported.

Diels–Alder cycloaddition reactions of 1,3,5-hexatriynes with tetraphenylcyclopentadienone under either thermal or microwave-assisted conditions give regioisomeric products that can be interpreted based on the size of the triyne end-groups.
08 Mar 10:18
by Jangwon Seo, Jun Hong Noh and Sang Il Seok

Accounts of Chemical Research
DOI: 10.1021/acs.accounts.5b00444

02 Mar 09:46
Chem. Commun., 2016, 52,4710-4713
DOI: 10.1039/C6CC01309K, Communication
Dominik Lungerich, Alexey V. Nizovtsev, Frank W. Heinemann, Frank Hampel, Karsten Meyer, George Majetich, Paul v. R. Schleyer, Norbert Jux
From a materials perspective, [18]annulene and its precursor reveal fascinating packing motifs in the solid state.
The content of this RSS Feed (c) The Royal Society of Chemistry

29 Feb 18:07
by Praveen K. Chinthakindi, Hendrik G. Kruger, Thavendran Govender, Tricia Naicker and Per I. Arvidsson

The Journal of Organic Chemistry
DOI: 10.1021/acs.joc.5b02770

23 Feb 14:55
by Ajay Ram Srimath Kandada and Annamaria Petrozza

Accounts of Chemical Research
DOI: 10.1021/acs.accounts.5b00464

Lucy, Hi and one other like this
23 Feb 14:54
by Jun Haruyama, Keitaro Sodeyama, Liyuan Han and Yoshitaka Tateyama

Accounts of Chemical Research
DOI: 10.1021/acs.accounts.5b00452

22 Feb 17:12
by Yong Jun Li, Yuanchao Lv, Chang-Ling Zou, Wei Zhang, Jiannian Yao and Yong Sheng Zhao

Journal of the American Chemical Society
DOI: 10.1021/jacs.5b12755

22 Feb 17:12
by Li Na Quan, Mingjian Yuan, Riccardo Comin, Oleksandr Voznyy, Eric M. Beauregard, Sjoerd Hoogland, Andrei Buin, Ahmad R. Kirmani, Kui Zhao, Aram Amassian, Dong Ha Kim and Edward H. Sargent

Journal of the American Chemical Society
DOI: 10.1021/jacs.5b11740

22 Feb 17:12
by Jonathan R. Petrie, Valentino R. Cooper, John W. Freeland, Tricia L. Meyer, Zhiyong Zhang, Daniel A. Lutterman and Ho Nyung Lee

Journal of the American Chemical Society
DOI: 10.1021/jacs.5b11713




 Open Access
Open Access