Shared posts
Chiral Redox-Active Isosceles Triangles
Highly Emissive Covalent Organic Frameworks
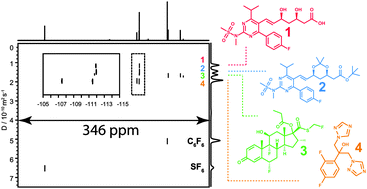
Very broadband diffusion-ordered NMR spectroscopy: 19F DOSY
DOI: 10.1039/C6CC02917E, Communication
 Open Access
Open Access   This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.
  This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.
A new pulse sequence, CHORUS Oneshot, allows measurements of diffusion-ordered spectroscopy (DOSY) spectra over the full chemical shift range of 19F for the first time.
The content of this RSS Feed (c) The Royal Society of Chemistry
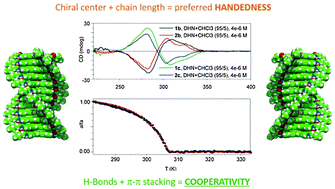
Helical supramolecular polymerization of C3-symmetric amides and retroamides: on the origin of cooperativity and handedness
DOI: 10.1039/C6CC02681H, Communication
N-centred trisamides 1 form helical aggregates with the same handedness than CO-centred trisamides 2 following a cooperative polymerization mechanism.
The content of this RSS Feed (c) The Royal Society of Chemistry
Covalent non-fused tetrathiafulvalene-acceptor systems
DOI: 10.1039/C6CC01827K, Feature Article
The main families of non-fused TTF-acceptors are discussed with a special focus on their characteristics and properties.
The content of this RSS Feed (c) The Royal Society of Chemistry
Copper-catalysed enantioselective stereodivergent synthesis of amino alcohols
Copper-catalysed enantioselective stereodivergent synthesis of amino alcohols
Nature 532, 7599 (2016). doi:10.1038/nature17191
Authors: Shi-Liang Shi, Zackary L. Wong & Stephen L. Buchwald
The chirality, or ‘handedness’, of a biologically active molecule can alter its physiological properties. Thus it is routine procedure in the drug discovery and development process to prepare and fully characterize all possible stereoisomers of a drug candidate for biological evaluation. Despite many advances in asymmetric synthesis, developing general and practical strategies for obtaining all possible stereoisomers of an organic compound that has multiple contiguous stereocentres remains a challenge. Here, we report a stereodivergent copper-based approach for the expeditious construction of amino alcohols with high levels of chemo-, regio-, diastereo- and enantioselectivity. Specifically, we synthesized these amino-alcohol products using sequential, copper-hydride-catalysed hydrosilylation and hydroamination of readily available enals and enones. This strategy provides a route to all possible stereoisomers of the amino-alcohol products, which contain up to three contiguous stereocentres. We leveraged catalyst control and stereospecificity simultaneously to attain exceptional control of the product stereochemistry. Beyond the immediate utility of this protocol, our strategy could inspire the development of methods that provide complete sets of stereoisomers for other valuable synthetic targets.
Directional Molecular Transportation Based on a Catalytic Stopper-Leaving Rotaxane System
A C60-aryne building block: synthesis of a hybrid all-carbon nanostructure
DOI: 10.1039/C5CC10462A, Communication
Covalent all-carbon few layer graphene and [60]fullerene conjugates can be easily formed from a versatile [60]fullerene-benzyne building block.
The content of this RSS Feed (c) The Royal Society of Chemistry
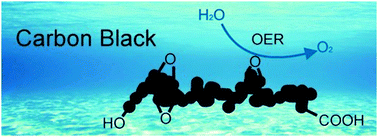
Surface-oxidized carbon black as a catalyst for the water oxidation and alcohol oxidation reactions
DOI: 10.1039/C6CC01319H, Communication
Surface oxidized carbon black after acidic treatment and electrochemical oxidation exhibits significant catalytic activity for the electrochemical oxidation of water and alcohols.
The content of this RSS Feed (c) The Royal Society of Chemistry
An autonomous molecular assembler for programmable chemical synthesis

Nature Chemistry. doi:10.1038/nchem.2495
Authors: Wenjing Meng, Richard A. Muscat, Mireya L. McKee, Phillip J. Milnes, Afaf H. El-Sagheer, Jonathan Bath, Benjamin G. Davis, Tom Brown, Rachel K. O'Reilly & Andrew J. Turberfield
Molecular machines that assemble polymers in a programmed sequence are fundamental to life. Now, synthetic machinery built from DNA has been used to execute a molecular program that produces peptides, or olefin oligomers, with a defined sequence. The oligomeric product is linked to a double-stranded DNA product that records the sequence of reactions that were executed.
Reversible transformation between ionic liquids and coordination polymers by application of light and heat
DOI: 10.1039/C6CC02807A, Communication
Reversible transformation between ionic liquids and coordination polymers by application of light and heat has been achieved.
The content of this RSS Feed (c) The Royal Society of Chemistry
Computer-Assisted Synthetic Planning: The End of the Beginning
By combining chemical knowledge with network theory and chess-like algorithms, computers can, at last, design synthetic pathways to non-trivial targets. The picture shows a cost-optimized synthesis of Taxol (large yellow node) selected by the Chematica program from amongst 400+ million possibilities in just 7 s. Red nodes are commercially available chemicals, blue are intermediates, green are side products, and yellow halos indicate regulated substances.
[Review]
Sara Szymkuć, Ewa P. Gajewska, Tomasz Klucznik, Karol Molga, Piotr Dittwald, Michał Startek, Michał Bajczyk, Bartosz A. Grzybowski
Angew. Chem. Int. Ed., April 08, 2016, DOI: 10.1002/anie.201506101. Read article
Hierarchical Self-Assembly of Polyoxometalate-Based Hybrids Driven by Metal Coordination and Electrostatic Interactions: From Discrete Supramolecular Species to Dense Monodisperse Nanoparticles
Self-limited self-assembly of nanoparticles into supraparticles: towards supramolecular colloidal materials by design
DOI: 10.1039/C6ME00016A, Minireview
We survey the most outstanding achievements on the rational design of supraparticles based on the self-limited self-assembly of nanoparticles.
The content of this RSS Feed (c) The Royal Society of Chemistry
Intramolecular transport of small-molecule cargo in a nanoscale device operated by light
DOI: 10.1039/C6CC02382G, Communication
A light-operated molecular nanodevice is able to transport an acetyl cargo intramolecularly over a distance of about 2 nm.
The content of this RSS Feed (c) The Royal Society of Chemistry
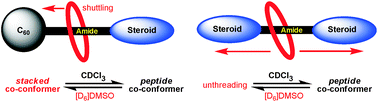
Diamide-based fullerosteroidal and disteroidal [2]rotaxanes: solvent-induced macrocycle translocation and/or unthreading
DOI: 10.1039/C6RA03872G, Paper
Two hydrogen bonded rotaxanes: a template directed synthesis, detailed characterization and shuttling/unthreading processes.
The content of this RSS Feed (c) The Royal Society of Chemistry
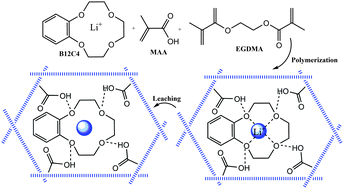
Synthesis of ion imprinted polymeric nanoparticles for selective pre-concentration and recognition of lithium ions
DOI: 10.1039/C5NJ03366G, Paper
A new Li+-IIP has been prepared for the fast determination and selective separation of lithium ions in aqueous samples.
The content of this RSS Feed (c) The Royal Society of Chemistry
Mono- and Ditopic Bisfunctionalization of Graphene
Two sides to the story: The use of two consecutive reduction and covalent addition steps leads to the monotopic and ditopic functionalization of individual graphene layers on substrates (one side blocked) as well as graphene in dispersion (both sides accessible).
[Communication]
Kathrin C. Knirsch, Ricarda A. Schäfer, Frank Hauke, Andreas Hirsch
Angew. Chem. Int. Ed., April 01, 2016, DOI: 10.1002/anie.201511807. Read article
Construction of Smart Supramolecular Polymeric Hydrogels Cross-linked by Discrete Organoplatinum(II) Metallacycles via Post-Assembly Polymerization
Crystal Fluidity Reflected by Fast Rotational Motion at the Core, Branches, and Peripheral Aromatic Groups of a Dendrimeric Molecular Rotor
A C216-Nanographene Molecule with Defined Cavity as Extended Coronoid
Self-Assembled Pyridine-Dipyrrolate Cages
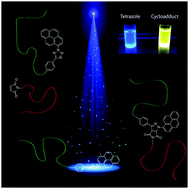
Catalyst free visible light induced cycloaddition as an avenue for polymer ligation
DOI: 10.1039/C6CC00942E, Communication
The current study introduces a tetrazole species able to perform a rapid, visible light induced nitrile imine-mediated tetrazole-ene cycloaddition (NITEC).
The content of this RSS Feed (c) The Royal Society of Chemistry
Self-assembly of size-controlled liposomes on DNA nanotemplates

Nature Chemistry. doi:10.1038/nchem.2472
Authors: Yang Yang, Jing Wang, Hideki Shigematsu, Weiming Xu, William M. Shih, James E. Rothman & Chenxiang Lin
Precise control of vesicle size is highly desirable both for basic biochemical research and biomedical applications. Now, monodispersed sub-100-nm vesicles with predefined sizes have been produced using a method based on membrane self-assembly within a DNA-nanostructure guide.
Synthesis and Properties of Endohedral Aza[60]fullerenes: H2O@C59N and H2@C59N as Their Dimers and Monomers
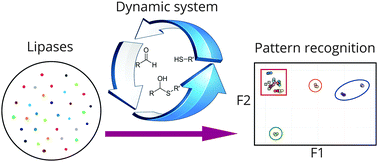
Enzyme classification using complex dynamic hemithioacetal systems
DOI: 10.1039/C6CC01823H, Communication
 Open Access
Open Access   This article is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported Licence.
  This article is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported Licence.
A complex dynamic hemithioacetal system was used in combination with pattern recognition methodology to classify lipases into distinct groups.
The content of this RSS Feed (c) The Royal Society of Chemistry
Light- and Heat-Triggered Reversible Linear–Cyclic Topological Conversion of Telechelic Polymers with Anthryl End Groups
Polymer nanocomposites from self-assembled polystyrene-grafted carbon nanotubes
DOI: 10.1039/C5NJ03285G, Paper
Supramolecular self-assembly and anisotropic patchiness generate long-range networks in polymer-grafted carbon nanotubes, opening new possibilities using industrially attractive processes.
The content of this RSS Feed (c) The Royal Society of Chemistry
VIP: Peptide [4]Catenane by Folding and Assembly
Dr. Tomohisa Sawada, Motoya Yamagami, Dr. Kazuaki Ohara, Prof. Dr. Kentaro Yamaguchi and Prof. Dr. Makoto Fujita
![Peptide [4]Catenane by Folding and Assembly Peptide [4]Catenane by Folding and Assembly](http://onlinelibrary.wiley.com/store/10.1002/anie.201600480/asset/image_n/anie201600480-toc-0001.png?v=1&s=353faf9bb61c5b89493027bb4c82cba848e8319e)
It's all about topology: Upon complexation with silver(I) the tripeptide ligand (Pro-Gly-Pro) simultaneously folds into an Ω-looped conformation and self-assembles into a discrete peptide [4]catenane with the crossing number of 12. Within the [4]catenane each of the four equivalent M3L3 macrocycles is interlocked with the other three rings with tetrahedral symmetry.
Dynamic foldamer chemistry
DOI: 10.1039/C6CC00788K, Feature Article
Dynamic foldamers translate chemical signals into conformational changes, and hence into chemical outputs such as control of reactivity and selectivity.
The content of this RSS Feed (c) The Royal Society of Chemistry