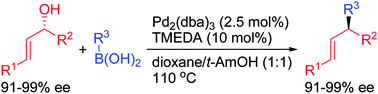06 Aug 06:24
Chem. Commun., 2015, 51,12680-12683
DOI: 10.1039/C5CC05125H, Communication
Jing Su, Bingqiang Wang, Dongling Liu, Libo Du, Yang Liu, Jihu Su, Wenjun Zheng
The oxidation of 1,2,4-diazaphospholide potassium (K+[2-]) produced a neutral 1,2,4-diazaphospholyl radical (2[radical dot]) that was able to self-associate through a N-P coupling to give a 2(N)-2(P) dimer.
The content of this RSS Feed (c) The Royal Society of Chemistry

09 Jul 05:26
by Haihui Peng, Novruz G. Akhmedov, Yu-Feng Liang, Ning Jiao and Xiaodong Shi

Journal of the American Chemical Society
DOI: 10.1021/jacs.5b05415

06 Jul 10:51
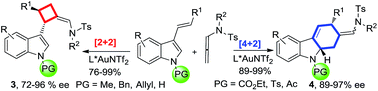
Chem. Sci., 2015, 6,5564-5570
DOI: 10.1039/C5SC01827G, Edge Article

Open Access
Yidong Wang, Peichao Zhang, Yuan Liu, Fei Xia, Junliang Zhang
The cycloaddition mode ([2+2] vs. [4+2]) can be unexpectedly switched by the simple modification of the N-substituent of the 3-styrylindoles.
The content of this RSS Feed (c) The Royal Society of Chemistry

30 Jun 15:00
by Shan Tang, Xudong Wu, Wenqing Liao, Kun Liu, Chao Liu, Sanzhong Luo and Aiwen Lei

Organic Letters
DOI: 10.1021/ol501584d

26 Jun 13:15
by Luca Deiana, Lorenza Ghisu, Samson Afewerki, Oscar Verho, Eric V. Johnston, Niklas Hedin, Zoltan Bacsik, Armando Córdova
Abstract
A modular design for a novel heterogeneous synergistic catalytic system, which simultaneously activates the electrophile and nucleophile by the combined activation modes of a separate metal and non-metal catalyst, for asymmetric cascade transformations on a solid surface is disclosed. This modular catalysis strategy generates carbocycles (up to 97.5:2.5 er) as well as spirocyclic oxindoles (97.5:2.5 to >99:0.5 er), containing all-carbon quaternary centers, in a highly enantioselective fashion via a one-pot dynamic relay process.

18 Jun 10:16
by Zhixun Wang, Yanzhao Wang and Liming Zhang

Journal of the American Chemical Society
DOI: 10.1021/ja503909c

14 Jun 16:53
by Scott E. Denmark and Hyung Min Chi

Journal of the American Chemical Society
DOI: 10.1021/ja5046296

30 Apr 11:16
by Malina Michalska, Olivier Songis, Catherine Taillier, Sean P. Bew, Vincent Dalla
Abstract
We report a unique mechanism-guided reaction that enhances and expands the chemical space that readily generated gold(I) acetylides currently operate in. Our strategy exploits the propensity of gold(I) carbophilic catalysts with specific counteranions (LAuX – X=triflate or triflimidate) to efficiently activate and desilylate trimethylsilylalkynes, thereby mediating the in situ formation of equal and catalytic quantities of a silyl Lewis acid (TMSX) of tunable strength and a nucleophilic gold(I) acetylide. This unprecedented manifold opens avenues for developing synergistic silyl-gold(I)-catalyzed alkynylation strategies of diverse pro-electrophiles which were heretofore unattainable, the proof of concept being principally exemplified herein with the first catalytic alkynylation of N,O-acetals. The reaction proceeds at low catalyst loading, employs mild reaction conditions, is easily scalable, and affords propargylic lactam products in good to excellent yields. Furthermore, it is fully amenable to a diverse array of structure and function substrates, and also expands to other pro-electrophiles beyond N,O-acetals. Control experiments have been carried out that strongly support our dual reaction mechanism proposal which, furthermore, itself outlines an inextricable link between the strength of the ancillary silyl Lewis acid (TMSOTf versus TMSNTf2) and the coordinating ability of the gold counter anion employed. This underlying feature of our system underscores its significant potential and flexibility, which indeed manifests with the demonstration that by carefully selecting the gold counter ion, it is possible to manipulate the strength of the ancillary silyl Lewis acid so that it can be tailored to the ionizing ability of a particular pro-electrophile.

26 Apr 11:36
by Bo Song, Lian-Hua Li, Xian-Rong Song, Yi-Feng Qiu, Mei-Jin Zhong, Ping-Xin Zhou, Yong-Min Liang
Abstract
A convenient zinc-promoted [4+3] cycloaddition of a carbonyl ene–yne with simple dienes was first achieved. This reaction provided an efficient strategy to prepare various cyclohepta[b]furan rings by cascade cycloadditions. Additionally, a multicomponent reaction of dione, alkynal, and diene was also reported, which exhibited a novel strategy for selective creations of C![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) O bonds and C
O bonds and C![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) C bonds.
C bonds.

Go tandem! A first zinc-catalyzed tandem [4+3] cycloaddition is presented herein. Various substituted cyclohepta[b]furans were synthesized from carbonyl ene–yne and diene in moderate to good yield. Furan rings and seven-membered rings were prepared in one single step (see scheme; TBS=tert-butyldimethyl).
26 Apr 11:33
by Kamil Parkan, Radek Pohl, Martin Kotora
Abstract
A convenient synthetic pathway enabling D-glucal and D-galactal pinacol boronates to be prepared in good isolated yields was achieved. Both pinacol boronates were tested in a series of cross-coupling reactions under Suzuki–Miyaura cross-coupling conditions to obtain the corresponding aryl, heteroaryl, and alkenyl derivatives in high isolated yields. This methodology was applied to the formal synthesis of the glucopyranoside moiety of papulacandin D and the first total synthesis of bergenin.

Building blocks with boron: A convenient synthetic route to D-glucal and D-galactal pinacol boronates was developed, and the boronates were used in cross-coupling reactions to generate the corresponding aryl, heteroaryl, and alkenyl derivatives in high yields (see scheme). This methodology was applied to the formal synthesis of the glucopyranoside moiety of papulacandin D and the total synthesis of bergenin.
24 Apr 08:37
by Yijin Su, Yanwei Zhang, Novruz G. Akhmedov, Jeffrey L. Petersen and Xiaodong Shi

Organic Letters
DOI: 10.1021/ol500854m

23 Apr 08:32
by Quan Chen, Thierry León, Paul Knochel
Abstract
We report a BF3-mediated direct alkynylation of pyridines at C(2) by using a variety of alkynyllithium reagents (oxidative cross-coupling). Moreover, we have developed a novel transition-metal-free cross-coupling method between alkylmagnesium reagents and 4-substituted pyridines, such as isonicotinonitrile and 4-chloropyridine, by employing BF3⋅OEt2 as a promoter. The combination of these methods enabled us to efficiently prepare a range of di-, tri-, and tetrasubstituted pyridines.

Oxidative or non-oxidative—That is the question! Pyridines bearing a substituent at position 4 readily undergo a BF3-mediated oxidative coupling at position 2 with a wide range of alkynyllithium compounds. In contrast, 4-cyano- or 4-chloropyridines undergo a novel BF3-mediated cross-coupling at position 4 with alkylmagnesium reagents. The combination of the two transition-metal-free procedures allows the preparation of a broad range of pyridines.
01 Apr 17:42
Chem. Commun., 2014, 50,4937-4940
DOI: 10.1039/C4CC00839A, Communication
Maria Camila Blanco Jaimes, Frank Rominger, Mariette M. Pereira, Rui M. B. Carrilho, Sonia A. C. Carabineiro, A. Stephen K. Hashmi
A turnover number of 28 000 000 can be achieved with this new type of gold(I) precatalysts bearing bulky phosphite ligands.
The content of this RSS Feed (c) The Royal Society of Chemistry

01 Apr 16:13
by Xiaoyu Yang, Robert J. Phipps and F. Dean Toste

Journal of the American Chemical Society
DOI: 10.1021/ja500882x

01 Apr 15:21
Chem. Soc. Rev., 2014, 43,2941-2955
DOI: 10.1039/C3CS60441A, Tutorial Review
Weibo Yang, A. Stephen K. Hashmi
In the last few years numerous new gold-catalysed reactions of allenes have been developed. The current mechanistic knowledge about different versatile reaction types like cycloadditions, hydroarylations, hydroalkoxylations and additions of carbonyl derivatives is based on experimental evidence and computational studies.
The content of this RSS Feed (c) The Royal Society of Chemistry

21 Mar 18:04
by Daniel Hack, Charles C. J. Loh, Jan M. Hartmann, Gerhard Raabe, Dieter Enders
Abstract
The combination of cinchona-alkaloid-derived primary amine and AuI–phosphine catalysts allowed the selective C![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) H functionalization of two adjacent carbon atoms of pyrroles under mild reaction conditions. This sequential dual activation provides seven-membered-ring-annulated pyrrole derivatives in excellent yields and enantioselectivities.
H functionalization of two adjacent carbon atoms of pyrroles under mild reaction conditions. This sequential dual activation provides seven-membered-ring-annulated pyrrole derivatives in excellent yields and enantioselectivities.

Outwitted: The combination of cinchona-alkaloid-derived primary amine and AuI–phosphine catalysts allowed the selective C![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) H functionalization of two adjacent carbon atoms of pyrroles under mild reaction conditions. This sequential dual activation provides seven-membered-ring-annulated pyrrole derivatives in excellent yields and enantioselectivities (see scheme).
H functionalization of two adjacent carbon atoms of pyrroles under mild reaction conditions. This sequential dual activation provides seven-membered-ring-annulated pyrrole derivatives in excellent yields and enantioselectivities (see scheme).
21 Mar 11:38
by Miles W. Johnson, Scott W. Bagley, Neal P. Mankad, Robert G. Bergman, Vincent Mascitti, F. Dean Toste
Abstract
The development of a gold(I)-catalyzed sulfination of aryl boronic acids is described. This transformation proceeds through an unprecedented mechanism which exploits the reactivity of gold(I)–heteroatom bonds to form sulfinate anions. Further in situ elaboration of the sulfinate intermediates leads to the corresponding sulfones and sulfonamides, two pharmacophores routinely encountered in drug discovery.

À la mode: A gold(I)-catalyzed synthesis of sulfinates has been accomplished. Preparation of proposed intermediates, X-ray studies, and a number of mechanistic experiments suggest that an unprecedented mode of reactivity for gold(I) has been achieved. The resulting sulfinates from this reaction can be functionalized in situ to form sulfones and sulfonamides.
17 Mar 19:37
Chem. Commun., 2014, 50,4130-4133
DOI: 10.1039/C4CC00739E, Communication
Jiabin Li, Kegong Ji, Renhua Zheng, Jonathan Nelson, Liming Zhang
Intermolecular trappings of in situ generated [small alpha]-oxo gold carbenes by allylic sulfides are enabled by a P,S-bidentate ligand.
The content of this RSS Feed (c) The Royal Society of Chemistry

03 Mar 12:00
Chem. Commun., 2014, 50,4272-4284
DOI: 10.1039/C4CC00072B, Feature Article
Yongming Deng, Siddhartha Kumar, Hong Wang
This review article highlights recent discoveries in synergistic-cooperative combination of enamine catalysis with transition metal catalysis.
The content of this RSS Feed (c) The Royal Society of Chemistry

25 Feb 13:05
by Luca Deiana, Yan Jiang, Carlos Palo-Nieto, Samson Afewerki, Celia A. Incerti-Pradillos, Oscar Verho, Cheuk-Wai Tai, Eric V. Johnston, Armando Córdova
Abstract
Herein is described a versatile and broad synergistic strategy for expansion of chemical space and the synthesis of valuable molecules (e.g. carbocycles and heterocycles), with up to three quaternary stereocenters, in a highly enantioselective fashion from simple alcohols (31 examples, 95:5 to >99.5:0.5 e.r.) using integrated heterogeneous metal/chiral amine multiple relay catalysis and air/O2 as the terminal oxidant. A novel highly 1,4-selective heterogeneous metal/amine co-catalyzed hydrogenation of enals was also added to the relay catalysis sequences.

Relay switch: A versatile strategy for expansion of chemical space and the synthesis of valuable molecules, having up to three quaternary stereocenters, in a highly enantioselective fashion from simple alcohols is described. The method employs integrated heterogeneous metal/chiral amine multiple relay catalysis and air/O2 as the terminal oxidant.
25 Feb 11:59
Chem. Soc. Rev., 2014, 43,3041-3105
DOI: 10.1039/C3CS60457H, Review Article
Elena Soriano, Israel Fernandez
This review covers the state-of-the-art applications of computational chemistry to understand and rationalize the bonding situation and vast reactivity of allenes.
The content of this RSS Feed (c) The Royal Society of Chemistry

09 Jan 09:28
Chem. Commun., 2014, 50,2420-2423
DOI: 10.1039/C3CC48869A, Communication
Rachel E. M. Brooner, Ross A. Widenhoefer
Bond length deformations within the cyclopropyl ring of a gold cyclopropyl(methoxy)carbene complex provide an experimental measure of the electron donor ability of the gold phosphine fragment.
The content of this RSS Feed (c) The Royal Society of Chemistry

09 Jan 09:18
Chem. Commun., 2014, 50,2275-2278
DOI: 10.1039/C3CC48508K, Communication
Amparo Sanz-Marco, Andrea Garcia-Ortiz, Gonzalo Blay, Jose R. Pedro
The first enantioselective conjugate alkynylation of [small beta]-trifluoromethyl [small alpha],[small beta]-enones using terminal alkynes and a taniaphos-Cu(I) complex as catalyst is described.
The content of this RSS Feed (c) The Royal Society of Chemistry

08 Jan 16:15
by Katrine Qvortrup, Danica A. Rankic and David W. C. MacMillan

Journal of the American Chemical Society
DOI: 10.1021/ja411596q

14 Dec 12:19
by Bojan Vulovic, Maja Gruden-Pavlovic, Radomir Matovic and Radomir N. Saicic

Organic Letters
DOI: 10.1021/ol4028557

10 Dec 09:23
Chem. Commun., 2014, 50,219-221
DOI: 10.1039/C3CC45772A, Communication
Hai-Bian Wu, Xian-Tao Ma, Shi-Kai Tian
Enantioenriched allylic alcohols underwent palladium-catalyzed cross-coupling with boronic acids in a stereospecific manner to give chiral alkenes with excellent ee.
The content of this RSS Feed (c) The Royal Society of Chemistry

02 Dec 09:10
by Adrien Quintard, Thierry Constantieux, Jean Rodriguez

Three is a lucky number: An enantioselective transformation of allylic alcohols into β-chiral saturated alcohols has been developed by combining two distinct metal- and organocatalyzed catalytic cycles. This waste-free triple cascade process merges an iron-catalyzed borrowing-hydrogen step with an aminocatalyzed nucleophilic addition reaction.
29 Nov 12:15
by Zhaofeng Wang, Xijian Li, Yong Huang
Abstract
Carbonyl-substituted allenes are highly important synthetic intermediates for a number of heterocycles and strained-ring systems. However, chemistry of allenyl aldehydes has not been explored as extensively as their ketone, ester, or amide analogues because of a lack of general synthetic methods. Described herein is the first direct α-vinylidenation of aldehydes and an α-vinylidenation/γ-functionalization cascade to access tri- and tetrasubstituted allenyl aldehydes using a combination of a gold catalyst and an secondary amine. The reactive enamine intermediate of an aldehyde reacts with the gold-activated hypervalent silylethynyl benziodoxolone to selectively generate the corresponding trisubstituted allenyl aldehyde. The allenyl aldehyde can further react with another equivalent of the alkynylation reagent or other electrophiles to afford tetrasubstituted allenes bearing an aldehyde group, an acetylene, and a halogen functionality. This method enables rapid access to polysubstituted furans from aldehydes.

Gold and amine team up: Gold and an amine catalyst work synergistically to promote either an α-vinylidenation or an α-vinylidenation/γ-functionalization of aldehydes to generate tri- and tetrasubstituted allenes. The allene products also undergo an additional reaction to generate polysubstituted furans.
25 Nov 15:21
by Masahiro Yoshida, Shoko Ohno, Kosuke Namba

Radical methods: The title reaction proceeds in the presence of a palladium catalyst to deliver substituted tetrahydrocyclobuta[b]benzofurans in a stereoselective manner (see scheme). A radical mechanism is discussed.
22 Oct 19:13
by Masanori Yoshida, Tatsuaki Terumine, Erika Masaki and Shoji Hara

The Journal of Organic Chemistry
DOI: 10.1021/jo4018414



 Open Access
Open Access





![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) O bonds and C
O bonds and C