Shared posts
Adhesive Photoswitch: Selective Photochemical Modulation of Enzymes under Physiological Conditions
UV-light-driven prebiotic synthesis of iron–sulfur clusters

Nature Chemistry. doi:10.1038/nchem.2817
Authors: Claudia Bonfio, Luca Valer, Simone Scintilla, Sachin Shah, David J. Evans, Lin Jin, Jack W. Szostak, Dimitar D. Sasselov, John D. Sutherland & Sheref S. Mansy
Current mineral-based theories do not fully address how enzymes emerged from prebiotic catalysts. Now, iron–sulfur clusters can be synthesized by UV-light-mediated photolysis of organic thiols and photooxidation of ferrous ions. Iron–sulfur peptides may have formed easily on early Earth, facilitating the emergence of iron–sulfur-cluster-dependent metabolism.
Nanoscale nuclear magnetic resonance with chemical resolution
Nuclear magnetic resonance (NMR) spectroscopy is a key analytical technique in chemistry, biology, and medicine. However, conventional NMR spectroscopy requires an at least nanoliter-sized sample volume to achieve sufficient signal. We combined the use of a quantum memory and high magnetic fields with a dedicated quantum sensor based on nitrogen vacancy centers in diamond to achieve chemical shift resolution in 1H and 19F NMR spectroscopy of 20-zeptoliter sample volumes. We demonstrate the application of NMR pulse sequences to achieve homonuclear decoupling and spin diffusion measurements. The best measured NMR linewidth of a liquid sample was ~1 part per million, mainly limited by molecular diffusion. To mitigate the influence of diffusion, we performed high-resolution solid-state NMR by applying homonuclear decoupling and achieved a 20-fold narrowing of the NMR linewidth.
How Simple Could Life Be?

Will we be ever able to produce living matter artificially? Despite our increasingly precise understanding of the details of life, its fundamental principles still lie in the dark. Armed with today's technology and knowledge about living systems, it is high time for us to re-address this persistent challenge in understanding nature. Graphics: Monika Krause, MPIB.
Switching between Anion-Binding Catalysis and Aminocatalysis with a Rotaxane Dual-Function Catalyst
Anion Recognition Strategies Based on Combined Noncovalent Interactions
Templated deprotonative metalation of polyaryl systems: Facile access to simple, previously inaccessible multi-iodoarenes
OleksandrMichael, have a look, may be you can find so,ething useful for your Gringard stuff
The development of new methodologies to affect non–ortho-functionalization of arenes has emerged as a globally important arena for research, which is key to both fundamental studies and applied technologies. A range of simple arene feedstocks (namely, biphenyl, meta-terphenyl, para-terphenyl, 1,3,5-triphenylbenzene, and biphenylene) is transformed to hitherto unobtainable multi-iodoarenes via an s-block metal sodium magnesiate templated deprotonative approach. These iodoarenes have the potential to be used in a whole host of high-impact transformations, as precursors to key materials in the pharmaceutical, molecular electronic, and nanomaterials industries. To prove the concept, we transformed biphenyl to 3,5-bis(N-carbazolyl)-1,1'-biphenyl, a novel isomer of 4,4'-bis(N-carbazolyl)-1,1'-biphenyl (CPB), a compound which is currently widely used as a host material for organic light-emitting diodes.
Integrative self-sorting of coordination cages based on 'naked' metal ions
DOI: 10.1039/C7CC03379F, Feature Article
In this review, we highlight recent approaches that facilitate integrative self-sorting of 'naked' metal ions and ligands to form multi-component, heteroleptic cage structures.
The content of this RSS Feed (c) The Royal Society of Chemistry
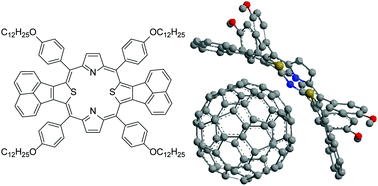
Polyaromatic molecular peanuts
Polyaromatic molecular peanuts
Nature Communications, Published online: 28 June 2017; doi:10.1038/ncomms15914
The complex, multicomponent structures often found in nature are difficult to mimic synthetically. Here, the authors assemble a molecular analogue of a peanut through coordinative and π-stacking interactions, in which a polyaromatic double capsule ‘pod’ held together by metal ions encapsulates fullerene ‘beans’.
Diastereoselective Self-Assembly of a Neutral Dinuclear Double-Stranded Zinc(II) Helicate via Narcissistic Self-Sorting
Abstract
A new bis(salicylimine) ligand based on the Tröger's base scaffold was synthesized in racemic and enantiomerically pure form. Upon coordination to zinc(II) ions this ligand undergoes highly diastereoselective self-assembly into neutral dinuclear double-stranded helicates as proven by XRD analysis and via comparison of experimental ECD spectra with those simulated with quantum-chemical methods. When the racemic ligand was used, self-assembly occurs under narcissistic self-sorting resulting in the formation of a racemic pair of helicates as revealed by NMR spectroscopy and XRD analysis.

A new bis(salicylimine) ligand based on Tröger's base scaffold was synthesized in racemic and enantiomerically pure form. Upon coordination to zinc(II) ions this ligand undergoes highly diastereoselective self-assembly into neutral dinuclear double-stranded helicates. Self-assembly of the racemic ligand occurs under narcissistic self-sorting, resulting in the formation of a racemic pair of helicates as revealed by NMR spectroscopy and XRD analysis.
A new and selective cycle for dehydrogenation of linear and cyclic alkanes under mild conditions using a base metal

Nature Chemistry. doi:10.1038/nchem.2795
Authors: Douglas P. Solowey, Manoj V. Mane, Takashi Kurogi, Patrick J. Carroll, Brian C. Manor, Mu-Hyun Baik & Daniel J. Mindiola
The direct, selective conversion of linear alkanes to α-olefins — without isomerization — is an important reaction in the field of catalysis from both a fundamental and industrial perspective. Now, a detailed pathway has been described for how a base metal can promote such a reaction with several turnovers, in a non-oxidative set of C–H activation reactions, thus preventing olefin isomerization.
Thermally bisignate supramolecular polymerization

Nature Chemistry. doi:10.1038/nchem.2812
Authors: Kotagiri Venkata Rao, Daigo Miyajima, Atsuko Nihonyanagi & Takuzo Aida
An attractive feature of supramolecular polymers is their reversibility — they typically depolymerize upon heating. Now, in the presence of a scavenger molecule, a metalloporphyrin derivative bearing eight amide-containing side chains has been shown to undergo supramolecular polymerization on heating as well as cooling through π-stacking and multivalent hydrogen-bonding interactions.
Dynamic Covalent Chemistry of Carbon Dioxide: Opportunities to Address Environmental Issues
Anion- and Solvent-Induced Rotary Dynamics and Sensing in a Perylene Diimide [3]Catenane
A New Wrinkle in Oligoarylene Architecture
Facile One-Pot Synthesis of Functional Giant Polymeric Vesicles Controlled by Oscillatory Chemistry
Abstract
We introduce a novel application of an oscillatory chemical reaction to the synthesis of block copolymers. The Belousov–Zhabotinsky (B-Z) reaction is coupled with the polymerization of an amphiphilic block copolymer. Radicals generated in the B-Z reaction initiate the polymerization between a polyethylene glycol (PEG) macroreversible addition-fragmentation chain-transfer agent and butyl acrylate monomers. The attachment of a hydrophobic block on PEG leads to self-assembly and formation of spherical micelles. The nanoscale micelles transform into submicrometer vesicles and grow to giant vesicles as a consequence of the oscillatory behavior of the B-Z reaction. The one-pot synthesis of an amphiphilic di-block copolymer and retention of oscillatory behavior for the B-Z reaction with the formation of giant vesicles bring a new insight into possible pathways for the synthesis of active functional microreactors in the range from hundreds of nanometers to tens of micrometers.

Get into the swing of things: An oscillatory chemical reaction is used to synthesize self-assembling active giant polymeric vesicles in one pot. The hydrophobic block on polyethylene glycol (PEG) undergoes self-assembly to form vesicles. In the scheme, the green squiggly line represents the PEG, the red star is the chain transfer agent (CTA), the yellow diamonds are components of the B-Z reaction, and the purple spheres are monomer units.
Common and Potentially Prebiotic Origin for Precursors of Nucleotide Synthesis and Activation
Semithiobambus[6]uril is a transmembrane anion transporter
DOI: 10.1039/C7CC04026A, Communication
Bambus[6]uril analogs are excellent anion binders but only the sulfur analog is also an effective anion transporter capable of polarizing lipid membranes through selective anion uniport.
The content of this RSS Feed (c) The Royal Society of Chemistry
Cobalt carbonate hydroxide superstructures for oxygen evolution reactions
DOI: 10.1039/C7CC04604A, Communication
A novel 3D superstructure of cobalt hydroxide carbonate with excellent electrocatalytic oxygen evolution reaction performance has been synthesized via a facile hydrothermal method.
The content of this RSS Feed (c) The Royal Society of Chemistry
[2]Rotaxane Formation by Transition State Stabilization
Kinetic Selectivity and Thermodynamic Features of Competitive Imine Formation in Dynamic Covalent Chemistry
Abstract
The kinetic and thermodynamic selectivities of imine formation have been investigated for several dynamic covalent libraries of aldehydes and amines. Two systems were examined, involving the reaction of different types of primary amino groups (aliphatic amines, alkoxy-amines, hydrazides and hydrazines) with two types of aldehydes, sulfobenzaldehyde and pyridoxal phosphate in aqueous solution at different pD (5.0, 8.5, 11.4) on one hand, 2-pyridinecarboxaldehyde and salicylaldehyde in organic solvents on the other hand. The reactions were performed separately for given amine/aldehyde pairs as well as in competitive conditions between an aldehyde and a mixture of amines. In the latter case, the time evolution of the dynamic covalent libraries generated was followed, taking into consideration the operation of both kinetic and thermodynamic selectivities. The results showed that, in aqueous solution, the imine of the aliphatic amine was not stable, but oxime and hydrazone formed well in a pH dependent way. On the other hand, in organic solvents, the kinetic product was the imine derived from an aliphatic amine and the thermodynamic products were oxime and hydrazone. The insights gained from these experiments provide a basis for the implementation of imine formation in selective derivatization of mono-amines in mixtures as well as of polyfunctional compounds presenting different types of amino groups. They may in principle be extended to other dynamic covalent chemistry systems.

Several dynamic covalent libraries of aldehydes and amines have been investigated with the focus on the kinetic and thermodynamic selectivities of imine formation. Two systems were examined, involving the reaction of different types of primary amino groups (aliphatic amines, alkoxy-amines, hydrazides and hydrazines) with two types of aldehydes, sulfobenzaldehyde and pyridoxal phosphate in aqueous solution at different pD on one hand, 2-pyridinecarboxaldehyde and salicylaldehyde in organic solvents on the other hand.
Adaptive self-assembly and induced-fit transformations of anion-binding metal-organic macrocycles
Adaptive self-assembly and induced-fit transformations of anion-binding metal-organic macrocycles
Nature Communications, Published online: 16 June 2017; doi:10.1038/ncomms15898
Container-molecules capable of recognizing charged species possess great potential as sensors, but are typically limited by their rigid frameworks. Here, Sun and co-workers design a family of adaptive metal-organic macrocycles that exhibit shape and size induced-fit transformations upon anion-binding.
Oxime-Based and Catalyst-Free Dynamic Covalent Polyurethanes
Calcium l-Lactate Frameworks as Naturally Degradable Carriers for Pesticides
Unraveling the Multistimuli Responses of a Complex Dynamic System of Pseudopeptidic Macrocycles

The understanding of increasingly complex chemical networks is a major challenge in Systems Chemistry. In this work, the effect of different stimuli on a dynamic combinatorial library of pseudopeptidic macrocycles has been analyzed using chemometrics. The approach is especially useful when several stimuli with overlapping effects are applied simultaneously (cover artwork by M. Eugenio Vázquez). More information can be found in the Full Paper by I. Alfonso et al. (DOI: 10.1002/chem.201701294).
Supramolecular functional assemblies: dynamic membrane transporters and peptide nanotubular composites
DOI: 10.1039/C7CC02997G, Feature Article
The fabrication of functional molecular devices constitutes one of the most important current challenges for chemical sciences.
The content of this RSS Feed (c) The Royal Society of Chemistry
Core expanded, 21,23-dithiadiacenaphtho[1,2-c]porphyrin interactions with [60]fullerene
DOI: 10.1039/C6NJ03353A, Letter
Saddle-shaped 21,23-dithiadiacenaphtho[1,2-c]porphyrin exhibits binding interaction with [60]fullerene in addition to photon absorption bands extending to 1000 nm.
The content of this RSS Feed (c) The Royal Society of Chemistry
Co-assembly of Peptide Amphiphiles and Lipids into Supramolecular Nanostructures Driven by Anion−π Interactions
Locked synchronous rotor motion in a molecular motor
Biological molecular motors translate their local directional motion into ordered movement of other parts of the system to empower controlled mechanical functions. The design of analogous geared systems that couple motion in a directional manner, which is pivotal for molecular machinery operating at the nanoscale, remains highly challenging. Here, we report a molecular rotary motor that translates light-driven unidirectional rotary motion to controlled movement of a connected biaryl rotor. Achieving coupled motion of the distinct parts of this multicomponent mechanical system required precise control of multiple kinetic barriers for isomerization and synchronous motion, resulting in sliding and rotation during a full rotary cycle, with the motor always facing the same face of the rotor.
Hot Paper: Chemically Controlled Spatiotemporal Oscillations of Colloidal Assemblies
Alicia Altemose, María Antonieta Sánchez-Farrán, Dr. Wentao Duan, Steve Schulz, Prof. Dr. Ali Borhan, Prof. Dr. Vincent H. Crespi and Prof. Dr. Ayusman Sen

Tunable colloidal oscillations: An autonomous oscillatory system involves Ag3PO4 colloidal particles under UV illumination in the presence of hydrogen peroxide. The particles oscillate between clustered and dispersed states, exhibiting signal propagation and synchronization. The behavior follows an electrolytic self-diffusiophoretic mechanism based on the redox chemistry of silver.