David Reger
Shared posts
Spiro-BODIPYs with a Diaryl Chelate: Impact on Aggregation and Luminescence
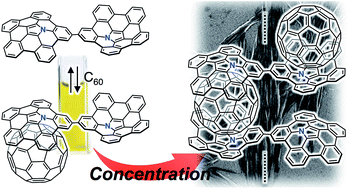
Supramolecular assemblies of a nitrogen-embedded buckybowl dimer with C60
DOI: 10.1039/C7SC04453D, Edge Article
 Open Access
Open Access   This article is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported Licence.
  This article is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported Licence.
A directly connected azabuckybowl dimer forms a 1 : 1 complex with C60 in a diluted solution, while 1D chain supramolecular assemblies are obtained upon increasing the concentration.
The content of this RSS Feed (c) The Royal Society of Chemistry
Fluorous photosensitizers enhance photodynamic therapy with perfluorocarbon nanoemulsions
DOI: 10.1039/C7CC07038A, Communication
Photodynamic therapy (PDT) requires a photosensitizer, light and oxygen to induce cell death. Here, we simultaneously deliver oxygen and photosensitizer using perfluorocarbon nanoemulsions.
The content of this RSS Feed (c) The Royal Society of Chemistry
D–π–A–π–D Dyes with a 1,3,2-Dioxaborine Cycle in the Polymethine Chain: Efficient Long-Wavelength Fluorophores
The nitrile group in newly synthesized 5-cyano-2,2-difluoro-4,6-dimethyl-1,3,2-dioxaborine is shown to significantly increase the acidity of the methyl groups. This allows the cyanine condensation with both methyl groups to form D–π–A–π–D dyes in which the dioxaborine cycle is a part of the polymethine chain. There, the reactivity of the remaining methyl group in the semi-product is reduced enough to perform the reaction stepwise, giving access to nonsymmetric derivatives. The synthesis of dyes with various electron donor termini [indole, benzothiazole, pyran, 4-(dialkylamino)phenyl] and with different polymethine chain lengths is reported. The obtained compounds show intense absorption and fluorescence in the red and NIR region, their fluorescence brightness (the product of the molar extinction and the fluorescence quantum yield) attaining a value of 200,000 m–1 cm–1. They are characterized by small-scale positive solvatochromism, yet their fluorescence quantum yield is much more sensitive to ambient polarity. Time-dependent (TD)DFT calculations have been performed to study the electronic structure of the new dioxaborines and reveal the nature of the long-wavelength electronic transitions in those molecules.

A newly synthesized 1,3,2-dioxaborine synthon comprises a 5-cyano group which significantly increases its activity in cyanine condensations. It gives access to new D–π–A–π–D functional compounds. These dyes intensely absorb and emit in the red and NIR region, their fluorescence quantum yield increasing in step with the electron-donating ability of the terminal groups.
DNA-Decorated Two-Dimensional Crystalline Nanosheets
Thiadiazoloquinoxaline-Fused Naphthalenediimides for n-Type Organic Field-Effect Transistors (OFETs)
Synthesis of Pyrrole-Fused Corannulenes: 1,3-Dipolar Cycloaddition of Azomethine Ylides to Corannulene
Along the rim: Reported is the 1,3-dipolar cycloaddition of a polycyclic aromatic azomethine ylide to corannulene, a reaction which occurs exclusively at the rim bond of corannulene, from the convex side in an exo fashion. The cycloadducts were converted, by oxidative dehydrogenation, into pyrrole-fused corannulenes, which exhibited pronounced solvatofluorochromism.
[Communication]
Yuki Tokimaru, Shingo Ito, Kyoko Nozaki
Angew. Chem. Int. Ed., November 13, 2017, https://doi.org/10.1002/anie.201707087 Read article
Edge Functionalization of Structurally Defined Graphene Nanoribbons for Modulating the Self-Assembled Structures
Diferrocenes Bridged by a Geminal Diethynylethene Scaffold with Varying Pendant Substituents: Electronic Interactions in Cross-Conjugated System
Exploring the Formation of Black Phosphorus Intercalation Compounds with Alkali Metals
Black to the future: The bulk intercalation of black phosphorus with alkali metals (namely: K and Na; black spheres) has been developed, showing a complex dynamic structural behavior. By in situ Raman spectroscopy and DFT calculations straightforward fingerprints for the identification of black phosphorus intercalation compounds (BPICs) have been demonstrated.
[Communication]
Gonzalo Abellán, Christian Neiss, Vicent Lloret, Stefan Wild, Julio C. Chacón-Torres, Katharina Werbach, Filippo Fedi, Hidetsugu Shiozawa, Andreas Görling, Herwig Peterlik, Thomas Pichler, Frank Hauke, Andreas Hirsch
Angew. Chem. Int. Ed., October 26, 2017, https://doi.org/10.1002/anie.201707462 Read article
Flash Vacuum Pyrolysis: Techniques and Reactions
Quick as a flash: This review describes flash pyrolysis methods for the synthesis of unusual molecules and for the characterization of reactive intermediates. The most important methods are presented, and an overview of typical reactions and observations that are possible with the various techniques are presented.
[Review]
Curt Wentrup
Angew. Chem. Int. Ed., October 25, 2017, https://doi.org/10.1002/anie.201705118 Read article
Regioselective Synthesis of [3]Naphthylenes and Tuning of Their Antiaromaticity
Red Light-Triggered CO Release from Mn2(CO)10 Using Triplet Sensitization in Polymer Nonwoven Fabrics
Supramolecular chemotherapy based on host-guest molecular recognition: a novel strategy in the battle against cancer with a bright future
DOI: 10.1039/C6CS00898D, Review Article
This review highlights the progress of supramolecular chemotherapy in cancer treatment based on host-guest interactions and provides guidance on the design of new targeting supramolecular chemotherapy combining diagnostic and therapeutic functions.
The content of this RSS Feed (c) The Royal Society of Chemistry
Direct Synthesis of Large-Scale Ortho-Iodinated Perylene Diimides: Key Precursors for Functional Dyes
Long-Range Orientational Self-Assembly, Spatially Controlled Deprotonation, and Off-Centered Metalation of an Expanded Porphyrin
4,5,9,10-Pyrene Diimides: A Family of Aromatic Diimides Exhibiting High Electron Mobility and Two-Photon Excited Emission
Conductive and emissive: Facile synthetic routes to reach the K-region of pyrene and produce 4,5,9,10-pyrene diimide (PyDI) derivatives are reported. The PyDI derivatives exhibited efficient electron transport properties, with the highest electron mobility of up to 3.08 cm2 V−1 s−1. The tert-butyl-substituted compounds (t-PyDI) also showed good one- and two-photon excited fluorescence properties.
[Communication]
Ze-Hua Wu, Zhuo-Ting Huang, Rui-Xue Guo, Chun-Lin Sun, Li-Chuan Chen, Bing Sun, Zi-Fa Shi, Xiangfeng Shao, Hanying Li, Hao-Li Zhang
Angew. Chem. Int. Ed., September 18, 2017, https://doi.org/10.1002/anie.201707529 Read article
Electron-Poor Bowl-Shaped Polycyclic Aromatic Dicarboximides: Synthesis, Crystal Structures, and Optical and Redox Properties
Synthesis of Phenalenyl-Fused Pyrylium Cations: Divergent C−H Activation/Annulation Reaction Sequence of Naphthalene Aldehydes with Alkynes
Shell out: The rhodium-catalyzed C−H activation/annulation sequence of naphthalene-type aldehydes with internal alkynes has been developed to provide access to phenalenyl-fused pyrylium cations. Also disclosed is the open-shell radical character of the reduced products.
[Communication]
Jiangliang Yin, Meiling Tan, Di Wu, Ruyong Jiang, Chengming Li, Jingsong You
Angew. Chem. Int. Ed., September 11, 2017, https://doi.org/10.1002/anie.201708127 Read article
Luminescent Carbon Dot Mimics Assembled on DNA
From Fenestrindane towards Saddle-Shaped Nanographenes Bearing a Tetracoordinate Carbon Atom
Warped nanographene: Two fourfold bay-bridged fenestrindane derivatives were prepared in good yields through a non-classical Scholl-type cyclization, paving the way to saddle-shaped nanographenes.
[Communication]
Wai-Shing Wong, Chun-Fai Ng, Dietmar Kuck, Hak-Fun Chow
Angew. Chem. Int. Ed., August 29, 2017, https://doi.org/10.1002/anie.201707505 Read article
The Rolling-Up of Oligophenylenes to Nanographenes by a HF-Zipping Approach
Abstract
Intramolecular aryl–aryl coupling is the key transformation in the rational synthesis of nanographenes and nanoribbons. In this respect the C−F bond activation was shown to be a versatile alternative enabling the synthesis of several unique carbon-based nanostructures. Herein we describe an unprecedentedly challenging transformation showing that the C−F bond activation by aluminum oxide allows highly effective domino-like C−C bond formation. Despite the flexible nature of oligophenylene-based precursors efficient regioselective zipping to the target nanostructures was achieved. We show that fluorine positions in the precursor structure unambiguously dictate the “running of the zipping-program” which results in rolling-up of linear oligophenylene chains around phenyl moieties yielding target nanographenes. The high efficiency of zipping makes this approach attractive for the synthesis of unsubstituted nanographenes which are difficult to obtain in pure form by other methods.

Roll up, roll up: Virtually linear fluorinated oligophenylenes are converted into the “preprogrammed” challenging nanographenes via effective domino-like aryl–aryl coupling. The fluorine positions in the precursor structure unambiguously dictate the running of the “HF-zipping-program” leading to the formation of the target nanographene structure.
Porphyrin Antennas on Carbon Nanodots: Excited State Energy and Electron Transduction
Antennas up! The synthesis and electron donor–acceptor features of a novel hybrid nanomaterial are reported. The nanohybrid combines the light-harvesting and electron-donating properties of a meso-tetraarylporphyrin (TArP) with the electron-accepting features of nitrogen-doped carbon nanodots (NCNDs).
[Communication]
Francesca Arcudi, Volker Strauss, Luka Đorđević, Alejandro Cadranel, Dirk M. Guldi, Maurizio Prato
Angew. Chem. Int. Ed., August 24, 2017, https://doi.org/10.1002/anie.201704544 Read article
Tetraalkoxyphenanthrene-Fused Hexadecadehydro[20]- and Tetracosadehydro[30]annulenes: Syntheses, Aromaticity/Antiaromaticity, Electronic Properties, and Self-Assembly
A meso–meso β-β β-β Triply Linked Subporphyrin Dimer
A triply linked subporphyrin dimer was synthesized by stepwise reductive elimination of a β-to-β doubly PtII-bridged subporphyrin dimer with the concurrent formation of the meso–meso bond. Despite the curved structure, the triply linked dimer displayed a remarkably red-shifted absorption spectrum and narrow electrochemical HOMO–LUMO gap because of the effectively conjugated π-electronic network.
[Communication]
Yasuhiro Okuda, Norihito Fukui, Jinseok Kim, Taeyeon Kim, Hua-Wei Jiang, Graeme Copley, Masaaki Kitano, Dongho Kim, Atsuhiro Osuka
Angew. Chem. Int. Ed., August 23, 2017, https://doi.org/10.1002/anie.201707123 Read article
Synthesis of Silicon and Germanium-Containing Heterosumanenes via Rhodium-Catalyzed Cyclodehydrogenation of Silicon/Germanium–Hydrogen and Carbon–Hydrogen Bonds
Direct Covalent Coupling of Porphyrins to Graphene
Metal-Free Oxidative C–C Coupling of Arylamines Using a Quinone-Based Organic Oxidant
Highly Efficient Chemiluminescence Probe for the Detection of Singlet Oxygen in Living Cells
Illuminating singlet oxygen: A chemiluminescence probe for singlet oxygen based on dioxetane formation is described. The dioxetane decomposes by a highly efficient chemiexcitation process to emit green light. The probe was used to detect and image intracellular 1O2 produced by a photosensitizer in HeLa cells during photodynamic therapy.
[Communication]
Nir Hananya, Ori Green, Rachel Blau, Ronit Satchi-Fainaro, Doron Shabat
Angew. Chem. Int. Ed., August 16, 2017, https://doi.org/10.1002/anie.201705803 Read article