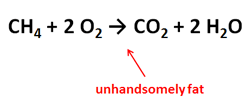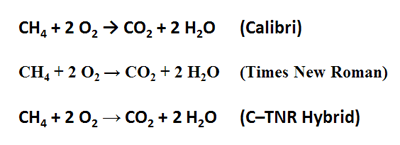Shared posts
Friday Fun: Meet Ginkgo Bioworks
Electric daisies: can a flower really give you a shock?
My Dad was visiting again the other day, making an absolutely lovely job of our garden as usual (our garden is beautiful, despite the fact that my last bit of flora-based input was sowing some grass seed six years ago), and we took a trip to the National Herb Centre. This is a wonderful place for a day trip if you’re in North Oxfordshire. Never mind the cafe, playground and interesting plants, it’s worth the trip just for the truly spectacular views.
We had lunch and wandered around the many, many rows of medical plants, dye plants and, of course, herbs. I had no idea there were that that many types of mint. I plumped for the chocolate one. Seriously, it’s a plant that smells of chocolate mint. How brilliant is that? I’m hoping for fruits in the form of After Eights. You never know.
But it was on the way out that things got really interesting. Dad spotted something and called me over to look at some fairly unprepossessing plants with little round, yellow flowers. A card described them as ‘electric daisies’ and explained that the flowers, when eaten, produce a sensation of an electric shock, or popping candy, and are used as a treatment for toothache and mouth ulcers.
Displaying my usual sort of ‘I wonder what will happen if I just mix this with that’ recklessness, I took a tentative nibble from one side of a flower. At first, it tasted of nothing so much as, well, pretty much how you might expect the middle bit of a daisy to taste: sort of grassy and mushy.
Then the weirdness began. A mild prickling sensation quickly developed into full-on tingling, but not big pins-and-needles tingling. It was a more subtle, evenly spread, effect. The best way I can describe it is like someone pouring lemon juice all over your tongue, having somehow first extracted the flavour of lemons. The peculiar, but not unpleasant, sensation persisted for a good ten minutes, and was followed by a strange rush of saliva. It was most odd.
It turns out that these little yellow flowers (technically, inflorescences) are chock full of interesting chemicals, most interestingly spilanthol which acts on the nerves in the mouth and causes saliva production. It’s also been shown to be antibacterial and anti-inflammatory, so this plant’s traditional use as a cure for toothache is based on a bit more than some in the ‘it might help and it can’t do any harm, can it’ school of plant remedies.
Interestingly, continuing my ongoing obsession of such things, it’s a chemical that actually does permeate skin, causing a local anaesthetic effect. And it can also, temporarily, stop muscles in your face from contracting – a bit like botox, but conveniently with less of the deadly toxin-ness. In fact, someone’s even registered a patent for a cosmetic treatment using spilanthol.
But why on earth has a plant evolved something that eases toothache and gets rid of wrinkles in humans? Perhaps not surprisingly, from the plant’s point of view, these effects are simply happy accidents. Spilanthol is a form of biological pest control – in other words it kills off critters that might be silly enough to try and make a snack of electric daisies. It’s particularly good at killing off yellow fever mosquitoes (mosquitoes eat plants when they’re not annoying you), which is good for both the plant and for humans since these little pests spread such delights as yellow fever and dengue fever.
Acmella oleracea flowers may not exactly give you an electric shock, but they can act as a painkiller, insect repellent and anti-ageing cure. Having learned a bit more about these little wonders I’m starting to think they might just be the most useful plant ever. Isn’t nature amazing?
You can buy electric daisies, and their seeds, at the National Herb Centre. I’m quite tempted to go back for another one actually…
S**t My Undergrads Say
I’m very fond of “ranking problems” that ask students to order a series of compounds in some way and provide an explanation. One of my all-time favorites is the famous “rank these acids from most to least acidic…” problem, which might include compounds like the following.
To really understand a problem like this deeply, the student has to be able to connect the structures provided with either physical/chemical properties or theoretical constructs (such as electron-donating and electron-withdrawing groups). Structure-property relationships are at the root of the appropriate thinking here. Unfortunately, as was pointed out by a recent paper that caught my attention in J. Res. Sci. Teach., students often struggle with structure-property relationships. Without experience under one’s belt, the incredible utility packed into a Lewis structure can be lost on students. It’s staggering, really—what other devices in science approach the information density of a chemical structure?!
I wanted to comment on this paper because it resonated with me. The authors explored the prior knowledge and heuristics that students used to rank the thermodynamic favorability of a series of chemical reactions. The work is a small piece of a larger movement that explores how chemists both novice and expert make judgments and decisions when solving problems.
I imagine that work in this field gets messy and discouraging very quickly. Novices are inclined to rely on unconvincing, arbitrary, or otherwise spurious assumptions. Is it worth drawing attention to these bogus ideas that guide students’ thought processes? That remains an open question, but I’d say that to conquer a problem, one must first hold it up to the light. As painful as it may be to learn just how little instruction affects students’ intuitive assumptions, “the first step is admitting you have a problem.” Chemistry experts, by the way, aren’t immune—just as many experts have trouble verbalizing their thought processes when navigating by feel!
In the paper, Maeyer and Talanquer studied problems related to acid-base chemistry, elemental synthesis, and combustion. They asked students to order sets of three related reactions from most favorable to least favorable in a thermodynamic sense. Qualitative interviews of a small set of students (33) supplemented large-scale survey data. The results were typical: a small number of students (less than 35%, generally) actively verbalized what the authors called valid chemical assumptions while rationalizing their orderings. Many more employed either intuitive assumptions that had at their core ideas completely unrelated to chemistry (“bigger things are harder to make than smaller things”) or spurious chemical assumptions with no basis in chemical theory but employing concepts from chemical theory (“electronegative atoms are more reactive”; bogus periodic trends).
With fascinating results, the authors also explored the heuristics students used to come to a decision. A majority of students focused on a single factor that they presumed to be the essence of the difference between the reactions—the authors called this the one-reason decision-making (ORDM) heuristic. The recognition heuristic played an important role, as many students stuck at the top or bottom of their lists reactions that involved familiar species (“Ooh, I’ve seen NaCl before, so its precipitation from water must be favorable”). Some students cited artificial correlations between variables, a strategy the authors called more A-more B (“Na to Ca to Al goes from +1 to +3, so I’m going to put them in this order”). Finally, the authors identified a representativeness heuristic that involved grouping items based on explicit similarities (“Cl and I in HCl and HI have the same charge, so I’m putting them next to each other in the list. S in H2S has different charge, so I’m putting it at the bottom of the list.”).
Painful to read, isn’t it? Many of the students generated correct orderings, but for all kinds of bogus reasons. Rather like buying your girlfriend flowers on Valentine’s Day because of a fight the night before…not exactly playing Casanova there! Of course, not all of the assumptions and reasoning strategies are bad. For example, ORDM reflects an analytical approach that teases out all the important differences between the reactions—the trick is weighing the differences against one another carefully instead of betting it all on a single factor.
Maeyer and Talanquer harbor no illusions concerning the generality of their work. Many times in their paper, as is typical of qualitative research articles in chemical education, they remark that the work used a small sample and lacks generality without further study. I think the work is still valuable for at least two reasons: (1) their framework of assumptions/heuristics provides a useful lens for diagnosing problems in the thought processes of students; and (2) it invites teachers young and old to take off their rose-colored glasses and reconsider how they teach. I don’t know about you, but I’d rather read about students’ issues with chemical reasoning before encountering it in my own classroom! This paper is one in a long line of studies that paint a descriptive picture of students’ chemical reasoning processes, and many more are to come. That’s “descriptive” as opposed to “prescriptive”: what “is” rather than what “ought to be” (although interestingly, Maeyer and Talanquer devote half a page to what ought to be in the form of the “target reasoning process”).
There are interesting parallels to ethics and philosophy here: should chemistry education researchers be focusing on what is rather than what ought to be? Why waste time on the way things are when we have so much to say to students about how they ought to think? Clearly, that “so much to say” doesn’t have as great an impact as we think it does—studies like this one demonstrate that convincingly. But will studying the way things are help us advance teaching and learning in a lasting way? I’m not sure. What do you think?
Here’s the full paper.
BSNYC Friday Fun Quiz!
Sophic.nealAll you need to read is the paragraph under the first picture (not the video, the picture).
If you answered, "Uhhh, I'm busy, Mom..." to any of these and then went back to your video game, Scion (Toyota's line of cars for young douchebags) has the sport coupe for you (via Twitter):
Yes, this is the car you need when you're "challenged by obstacles." And by "obstacles" they don't mean your mental handicap. No, by "obstacles" they mean cyclists:
"Faggy" cyclists wearing "Spandex" and Zach Galifianakis beards got you down? Simply grab your faux-crabon Phallo-matic™ shifter and experience the thrill of flirting with vehicular manslaughter:
Then thrill to sensation of those 179 horses galloping under the hood, which is almost enough to make you forget you'll never know a lover's touch:
You'll arrive at the "boxing gym" in style and ready to go:
And by "ready to go" I mean "with a semi-erection in your Ed Hardy underpants."
Speaking of bikes and fighting, some "fitness guru" thinks that instead of bike share we should have mixed martial arts share:
Would you rather take your chances participating in a city-backed transportation program, or in an illegal cage fight?
Participate in an activity that was responsible for nearly 700 American deaths in 2011 alone, or an activity that has been responsible for eight or nine fatalities in the past 20 years?
The former options, and latter options, on both of these questions are one in the same: Nearly 700 Americans were killed in bicycle accidents in 2011 alone, and, at most, nine fighters have died from Mixed Martial Arts since 1993 (only three of which have resulted from legitimate, sanctioned bouts).
Is this guy actually comparing a widely used form of transportation to a competitive fighting discipline that appeals to people who drive Scions with automatics? Why? What does one have to do with the other? Do a lot of people wake up in the morning and ask themselves, "Hmmm, should I take the subway to work this morning, or should I just kung fu my way to the office? Well, statistically kung fu is safer so enter the dragon, motherfucker!" I mean, come on, it's not even apples and oranges. It's apples and space boogie disco monkeys
Still, I guess he thinks Citi Bike vs. mixed martial arts is a valid comparison since he's gotten hurt while doing both:
As someone who values science, statistics and data, and who has been hit by cars while biking, by bikes while walking and by punches in the ring, I can tell you that I would rather take my chances with the latter any day. Unfortunately those in New York City walking, biking and commuting to work don't have this choice. They'll simply have to trust that Citi Bike and City government has their best interests at heart, m'kay?
Look, I support his assertion that mixed martial arts should be legal. After all, douchebags need entertainment too. Still, he should really be careful, because he's one punch away from hitting "Full Rabinowiz" on the Scale of Dementia:
As for the high valuation he places on science, clearly that's something he shares in common with the Insane Clown Posse.
And now, I'm pleased to present you with a quiz. As always, study the item, think, and click on your answer. If you're right that's great, and if you're wrong you'll see douchebags.
Thanks very much for reading, ride safe, and always wear your helment when martial arting to work.
--Wildcat Rock Machine
1) This man is:
--"Schluffing"
--"Schlurfing"
--"Smurfing"
--Performing an advanced Dutch bike freestyle move called "Threading the Flagel"
2) In a hard-hitting story on cycling fashion, the New York Times reveals that:
--Walking shoes make good riding shoes
--Something about flip-flops and Dairy Queen
--Wrestling with a Citi Bike is a great way to work your muscles
--All of the above
3) In what will surely be the biggest boon to helment sales since Freds started falling for the whole "you need to replace it every two years or it goes bad" thing, a hard-hitting New York Times story reveals that, “Similar to a handbag or shoes, you don’t necessarily have to wear the same helmet every day.”
--True
--False
4) Seriously, a fucking crib at brunch?
--Sadly, yes.
--Thankfully, no.
5) How much does money someone on Kickstarter want in order to build a panda out of bike tires?
--$250
--$2,500
--$25,000
--No money, just bamboo
6) According to the Wall Street Journal, cargo bikes are:
--"The new station wagon"
--"The new SUV"
--"The new smugness"
--"Begriming the city's most beautiful neighborhoods"
7) Cabbage is delicious.
--Yes
--No
Overtime
Sophic.nealI want to post this at my work desk. It might get me sacked.
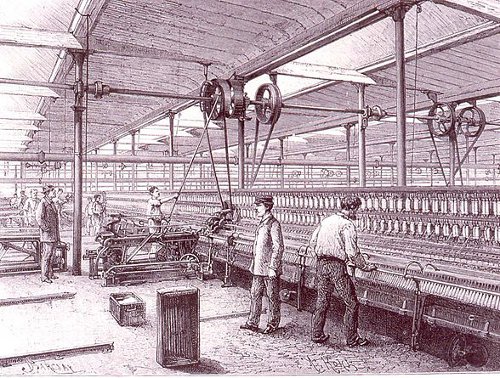
Suppose that, at a given moment, a certain number of people are engaged in the manufacture of pins. They make as many pins as the world needs, working (say) eight hours a day. Someone makes an invention by which the same number of men can make twice as many pins: pins are already so cheap that hardly any more will be bought at a lower price. In a sensible world, everybody concerned in the manufacturing of pins would take to working four hours instead of eight, and everything else would go on as before. But in the actual world this would be thought demoralizing. The men still work eight hours, there are too many pins, some employers go bankrupt, and half the men previously concerned in making pins are thrown out of work. There is, in the end, just as much leisure as on the other plan, but half the men are totally idle while half are still overworked. In this way, it is insured that the unavoidable leisure shall cause misery all round instead of being a universal source of happiness. Can anything more insane be imagined?
– Bertrand Russell, “In Praise of Idleness,” 1935
Can you get drunk through your toes?
Can you get drunk by dunking your feet in alcohol? A strange question you might think, but an interesting one. I recently wrote a post responding to some rather outlandish claims made on the Jeremy Vine radio show, and one of them was that we absorb 14 kg of toxins annually through our skin into our bloodstream. This one was so questionable that I started a quest to Ask for Evidence (a campaign run by the charity Sense About Science) on the subject. It’s thrown up a up a number of interesting bits and pieces, and there will be more to come on this topic.
 But in the meantime, absorption of chemicals through skin was on my mind as I was listening to the Ask the Naked Scientists podcast. A question about methylated spirits came up. In his answer, Dr Chris Smith referred to a rather brilliant piece of work by some Danish scientists.
But in the meantime, absorption of chemicals through skin was on my mind as I was listening to the Ask the Naked Scientists podcast. A question about methylated spirits came up. In his answer, Dr Chris Smith referred to a rather brilliant piece of work by some Danish scientists.
It was published in the British Medical Journal, and here’s the title: Testing the validity of the Danish urban myth that alcohol can be absorbed through feet: open labelled self experimental study
Now if that doesn’t make you want to read on, I don’t know what will. It would appear that along with stories of suicidal architects and families being duped on holiday, there is a popular Danish urban legend that you’ll become drunk if you submerge your feet in alcoholic drink.
So late in 2010 three researchers – you can listen to an interview with one of them here - decided to test this theory, using themselves as subjects (three isn’t a very robust sample size, but perhaps they didn’t have the resources to recruit more volunteers – vodka is expensive after all).
They abstained from alcohol for 24 hours before the test to ensure that there was none in their blood, and carefully exfoliated their feet with loofas to remove dry skin. Their blood was monitored through a venous line and a ‘before’ blood alcohol level was recorded.
And then they submerged their feet in washing up bowls filled with the contents of three 700 mL bottles of 37.5% alcohol vodka, for three hours.
What happened? Sadly, very little. Their blood alcohol levels stayed below the detection limit for the whole three hours. They didn’t get drunk, their self-confidence didn’t suddenly improve, they didn’t become noticeably more chatty and no one had the urge to spontaneously hug anyone else (all these things were monitored).
It seems fairly conclusive that you can’t get drunk through your skin. Now, the alcohol is vodka is ethanol, C2H5OH. It’s quite a small molecule, and if it can’t get into your bloodstream when you submerge your feet right in it, then I think that really does call into question the likelihood that 14 kg of ‘toxins’ are sneaking past our skin’s defences every year.
The researchers, by the way, published their work in December 2010, and called it the: Percutaneous Ethanol Absorption Could Evoke Ongoing Nationwide Euphoria And Random Tender Hugs study. Who says scientists don’t have a sense of humour?
DNA and RNA “Traffic Lights”: Synthetic Wavelength-Shifting Fluorescent Probes Based on Nucleic Acid Base Substitutes for Molecular Imaging
Sophic.nealTom, would this actually work?
About.com Just About Makes Me Vomit
Whenever you search for any standard do-it-at-home chemistry demo, most of the time you will come across an entry on About.com. About.com is a site that tries to gobble up pageviews so it can attract advertisers, and one method they use for doing this is to pay “guides” to write about popular topics within a particular subject area.
The guide for chemistry is Anne Marie Helmenstine, who earned a Ph.D. in biomedical sciences from the University of Tennessee. Probably sensing an opportunity to gobble up pageviews, Dr. Helmenstine jumped on the liquid nitrogen pool party story. If all she wanted to do was attract hits, that was an excellent plan. Yesterday alone, ChemBark got 21k pageviews, which is about 7-10 times what the site gets on a typical day with a fresh post. But, if your job is to educate and inform, I would not recommend writing this:
Nitrogen is principal gas in air, so it it’s quite safe on its own, but the liquid nitrogen was believed to have reacted with treatments in the pool, releasing toxic gas. How do you avoid hospitalizing your party, if you want liquid nitrogen fog? Simply add the nitrogen to ordinary water, not chemical-treated water. You’ll get fog without additional compounds. The principal risk from liquid nitrogen fog is from asphyxiation. Adding more nitrogen to the air decreases the relative amount of oxygen. This isn’t an issue so much in an outdoor pool, but should be a consideration if you have an enclosed pool. It’s safer to use a fog machine or make real water-based fog in that situation.
That is Dr. Helmenstine’s expert advice, and it is atrocious. First, there is very little chance the nitrogen reacted with the hypochlorite in the pool. Second, adding the liquid nitrogen to non-chlorinated water would have posed the same danger to life as adding it to chlorinated water. Finally, to write that this “isn’t an issue so much in an outdoor pool” is not only wrong, it is a public health hazard. The pool party disaster took place at an outdoor pool.
Ugh!
Wow Looks Like Someone Encased A Tiny Alive Person Entirely In Eggshell And Then Sat On That Person Until They Escaped: Parenthood For Dinosaurs 202
| archive - contact - sexy exciting merchandise - cute - search - about | |||
| dinosaur comics returns monday! :o
|
|||
| ← previous | June 20th, 2013 | next | |
|
June 20th, 2013: Have you watched my TEDxUofT talk? No? But what if I told you it was... on how to use time travel to become A GOD IN THE PAST??
One year ago today: other flawless idea: autoreply with "i will answer for $5", every time you get a positive response, reply with "the answer is now [$previousamount + $5], do you agree??] – Ryan 
| |||
According to Wikipedia, "...The Goose That Laid the Golden Eggs is among the best known of Aesop's Fables (Perry 87) and use of the phrase has become idiomatic of an [excellent scientific investigation] motivated by [the desire to better understand the un
| archive - contact - sexy exciting merchandise - cute - search - about | |||
| dinosaur comics runs mon / wed / fri this week due to me kiiiinda signing thousands and thousands of books!
|
|||
| ← previous | June 24th, 2013 | next | |
|
June 24th, 2013: I am in Austin, Texas! So far I haven't done much but sign books! It is a very busy trip to Austin, Texas! One year ago today: wrote this comic while finding a series of ants crawling up my legs, not even joking – Ryan 
| |||
There’s rat poison in my milk? or, why everything is toxic (even Kombucha tea)
Sue the “Chemical-Free” Bastards
 While I’m an idealist at heart, in my old age, I’ve increasingly found myself giving in to apathy and letting things slide more often. In the shower this weekend, I saw another one of my fiancée’s hair-care products advertised as “chemical free” and was soon awash in the same frustration expressed in this old post. The phrase “chemical free” is profoundly stupid, it is damaging to our field, and the problem is getting worse. While a lot of frustration has been expressed by a small group of chemists online, I’ve seen no effective campaign against the stupidity of the term “chemical free.” On the flip side, you’ve got another group of chemists who find the argument against “chemical free” to be pedantic, and some have even embraced the term for use in lab.
While I’m an idealist at heart, in my old age, I’ve increasingly found myself giving in to apathy and letting things slide more often. In the shower this weekend, I saw another one of my fiancée’s hair-care products advertised as “chemical free” and was soon awash in the same frustration expressed in this old post. The phrase “chemical free” is profoundly stupid, it is damaging to our field, and the problem is getting worse. While a lot of frustration has been expressed by a small group of chemists online, I’ve seen no effective campaign against the stupidity of the term “chemical free.” On the flip side, you’ve got another group of chemists who find the argument against “chemical free” to be pedantic, and some have even embraced the term for use in lab.
While I might be closer to abandoning this cause in favor of apathy, I don’t think the problem is worth giving up on just yet. Previously, I have lobbied bench chemists to become more involved in educating the public, suggested that the ACS develop a “war room” to address misinformation in the mainstream media, and volunteered a first draft of a pro-chemicals ad campaign. But maybe that’s my idealism talking. “Positive” approaches, where we tout the benefits of chemistry, require a lot of effort and take time to bring about change. Perhaps it would be more effective to adopt a negative approach? And, of course, what approach could be more negative than to sue?
SUE, SUE, SUE!
Yes, let’s start suing companies that advertise their products as “chemical free”. Make the dummies hurt where it counts: their wallets.
You see, there are laws that require truth-in-advertising, and many of them should be directly applicable to “chemical free” cases. The Federal Trade Commission has explained its policy for how it judges whether or not it will act on potentially deceptive ads:
Certain elements undergird all deception cases. First, there must be a representation, omission or practice that is likely to mislead the consumer. Practices that have been found misleading or deceptive in specific cases include false oral or written representations, misleading price claims, sales of hazardous or systematically defective products or services without adequate disclosures, failure to disclose information regarding pyramid sales, use of bait and switch techniques, failure to perform promised services, and failure to meet warranty obligations.
Second, we examine the practice from the perspective of a consumer acting reasonably in the circumstances. If the representation or practice affects or is directed primarily to a particular group, the Commission examines reasonableness from the perspective of that group.
Third, the representation, omission, or practice must be a “material” one. The basic question is whether the act or practice is likely to affect the consumer’s conduct or decision with regard to a product or service. If so, the practice is material, and consumer injury is likely, because consumers are likely to have chosen differently but for the deception. In many instances, materiality, and hence injury, can be presumed from the nature of the practice. In other instances, evidence of materiality may be necessary.
Thus, the Commission will find deception if there is a representation, omission or practice that is likely to mislead the consumer acting reasonably in the circumstances, to the consumer’s detriment.
So, there are three major criteria for an ad to meet: (i) it must be deceptive, (ii) it must have an impact on a “reasonable” consumer, and (iii) it must induce a material change in behavior on the part of a consumer. Any product which advertises itself as “chemical free” easily meets these three criteria.
First, everything that I have come across as “chemical free” (e.g., cosmetics, fertilizer, sunscreen) has contained chemicals, so the claim is a false written representation of the product. Second, the fear of chemicals is so widespread in society that it is more than reasonable for an average, uneducated consumer to care whether something is “chemical free”. Finally, a “chemical free” label can easily have a material effect in that a chemophobic consumer could choose to purchase a falsely labeled product over a competing product that is not labeled “chemical free” yet contains the same active chemicals.
Unfortunately, it is probably unreasonable for any halfway-decent chemist to claim he was fooled by something as scientifically nonsensical as “chemical free.” But, a class-action lawsuit waged by a group of typical (chemophobic) consumers who’ve purchased a chemical-free product could easily have merit.
Perhaps even more effective would be for a major company (with hefty legal resources) to file lawsuits claiming damages due to unfair business practices. It is unfair to have to compete against rival products that benefit from advertising with false claims. Let’s see these smaller companies have to defend themselves against the giants. Unfortunately, there’s no single chemical-free product on the market that is successful enough to make such a lawsuit a worthwhile cause.
Outside of lawsuits, what can you do? Well, the FTC has an FAQ about what sorts of ads are potentially deceptive, and you can contact them to register a complaint about products making false claims. Some complaints they pursue and some they don’t, but their decisions carry the weight of the law and they can issue cease-and-desist orders as well as fines. You can also register complaints with the Better Business Bureau (BBB) and state/local consumer protection offices.
I’m not saying that lawsuits are the best approach to solving the problem, but in some cases, insisting on the enforcement of existing laws might be an effective solution to the proliferation of all this “chemical free” nonsense.
Awful Idea: Liquid Nitrogen at a Pool Party
There is video circulating tonight of a summer party in Mexico where organizers poured liquid nitrogen into the swimming pool. They weren’t messing around, either—you can see a number of ~10 L dewars being upended into the water.
--Of course, this is a very, very bad idea. Under these conditions, the nitrogen (b.p. = −196 °C) quickly boils and displaces all of the oxygen present, causing people in the area to asphyxiate. Remember, it only takes 28 g of liquid nitrogen to displace 22.4 L of air at standard temperature and pressure. The fact that you are asphyxiating is masked by your ability to exhale carbon dioxide normally, so the burning sensation you experience when holding your breath or drowning is absent. Furthermore, the thick fog produced upon mixing the liquid nitrogen and water conceals any distress.
It looks like multiple people were hurt at the party and needed to be rescued.
News reports, updated 21 June 2013: Daily Mail (corrected most of the wrong chemistry, but a caption referencing a reaction with chlorine remains), Latin Rapper Blog (corrected to get the chemistry right; gives ChemBark a shout out, see comments), International Science Times (gets it right, credits ChemBark), Excelsior (gets the chemistry wrong), Fox News (gets the chemistry wrong), MSN Now (gets the chemistry wrong), Huffington Post UK (gets chemistry mostly right, credits ChemBark, but still needs to correct photo captions), NY Daily News (gets the chemistry wrong), The Sun (gets the chemistry wrong), The Telegraph (gets the chemistry wrong in the headline), Popular Science (gets the chemistry right, includes interview and link to ChemBark), Slate (corrected the story to get the chemistry right).
Deborah Blum covered the misreports by the media of the chemistry at play on the Knight Science Journalism (KSJ) site. The post links to ChemBark.
Updated note to media: Many outlets have reported that liquid nitrogen will react with chemicals in swimming pools to generate a poisonous gas. This is almost certainly incorrect. Molecular nitrogen is relatively inert and should not react with anything present in the pool, like “chlorine” (mostly, NaOCl) or water. The danger of adding liquid nitrogen to the pool stems simply from the nitrogen’s boiling and pushing away all of the oxygen around, leaving none for the swimmers to breathe. The cloud that is present has nothing to do with poison gas. Rather, it is the same thing as fog. The liquid nitrogen is cold and cools the air close to the pool to the point that water vapor in the air condenses into very tiny droplets that stay suspended. The cloud is fog, generated from the same effect responsible for your being able to “see your breath” when you exhale on a cold day. The visible cloud is not nitrogen and it is not smoke; it is droplets of water suspended in an atmosphere of air that is heavily enriched in nitrogen.
BuLi: Not Just a Base Anymore
What about using BuLi as an off-the-shelf cross-coupling partner? Sure, sounds like a methyl-ethyl-butyl-futile joke gone too far, but there're quite a few of those aryllithiums in catalogs nowadays...
Looks like Ben Feringa's got you covered. In Nature Chemistry, his group reveals that slow addition of a diluted organolithium to an aryl or vinyl bromide mixed with some electron-rich Pd(0) precatalysts provides alkylated products with high selectivity. At room temp. In one hour. In the presence of chlorides, esters, free alcohols, and all sorts of things that usually gum up the works. Zoinks!
I'll admit it - As someone who's spent a decent part of my life trying to glom one carbon onto another (grumbling while I purified yet another organotin or boronate), these initial results look promising. I'm sure they're furiously working on the alkyl-alkyl (sp3) coupling as we speak - good luck, that one's gonna be a lot harder.
P.S. Kudos for a well-thought-out Supporting Info section, too. The authors discuss their optimization process almost "stream-of-consciousness"-like, letting you vicariously learn the chemistry along with them.
Back to the Basics – Dispensing Solids and Liquids
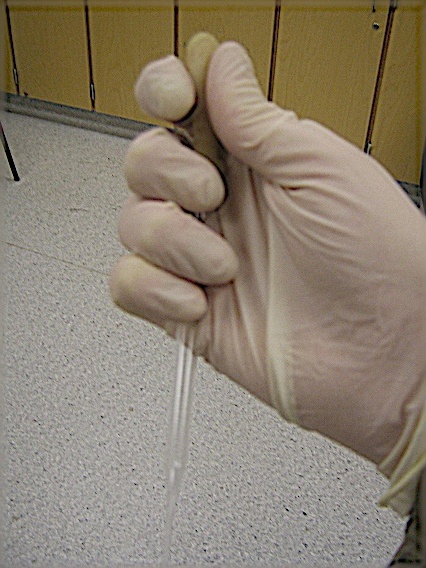
“How do you properly hold a spatula/scoopula or a Pasteur pipette?” isn’t a question I’m often asked on this blog [1], but it is something I’m a little pedantic about.
Pasteur pipettes are best gripped with the bottom three fingers of your hand, leaving the index finger and thumb free to press in the bulb. Unlike the more natural “thumb and forefinger on the bulb” grip, this keeps the tip of the pipette almost perfectly still, giving you a lot more control over where and when the liquid will be dispensed. As the bulb is no longer supporting any weight the pipette is also harder to accidentally squeeze, a problem with the bulb-only grip.
Proper spatula/scoopula technique is a little less well known, as the advantages aren’t quite as obvious. Similar to the pipette, the bottom three fingers of your hand should do most of the gripping, with the added addition of your thumb serving as an upper counterweight. Your index finger is kept free to tap the side of the spatula/scoopula, allowing gentle dispension of whatever solid is loaded. By shifting the angle of the scoopula/spatula more or less solid will be shaken free with each tap. With a little practice measuring solids will speed up three or four fold, as it’s now easy to sprinkle the exact amount of solid you need onto a weigh boat.
![The angle of the scoopula determines drop rate [2].](http://chemtips.files.wordpress.com/2013/05/img_0671-rotate.jpg?w=529&h=397)
The angle of the scoopula determines drop rate [2].
[1] I don’t get all that many questions, to be honest. If you’ve got something you’d like me to discuss, send me an email (brandon [dot] findlay [at] zoho [dot] com), leave a comment somewhere on Chemtips, or meet me on Twitter.
[2] Fun fact? Holding a scoopula in one hand while photographing it with the other is extremely awkward. Please forgive the strange angle.
Filed under: How it's Done
Welcome to the Lab
Over on ChemBark, Paul and co. have been talking about welcome packages, the formal documents given to incoming students to make them acquainted with lab culture, duties and equipment. The discussion so far has focused on lab culture and unwritten rules, like the notes emphasizing the importance of bathing and guidelines for asking for a reference letter. However, what’s caught my interest is (of course) the groups’ standard operating procedures.
One of the hazards of a long-running lab is degradation of the “institutional knowledge”. Learning a new technique alone can require exhaustive reading or extensive trial and error, but once mastered it’s a relatively simple process to teach other members of the lab [1]. Over time everyone in the lab becomes proficient in the common techniques (flash chromatography, 2D NMR analysis, etc.), but more specialized skills often remain the domain of a single student. When that person graduates the knowledge is effectively lost.
These welcome packages (and other, less public documents) are an attempt to retain information from the best and brightest of days gone by, while cutting down on the amount of time spent on simple training. Not surprisingly, much of the details aren’t widely applicable [2], but the readers of Chembark have turned up some real gems. I’ll highlight my picks below; leave a link if you have a favourite I’m leaving out.
1) Using the Schenk Line and Glovebox in the Bartlett Lab (PDF, pg 12-21)
Dr. Bartlett only set up his lab in 2009, but has already put together a comprehensive booklet for incoming students. There’s a strong focus on anhydrous/anoxic techniques, and I found the glovebox section in particular quite informative. Points for being one of the few welcome package to include colour photos.
2) GC Yield Determination in the Watson Lab (PDF, pg 13)
This forms a nice complement to the NMR yield determination method I highlighted last August. There’s a few additional steps for converting standard peaks to a true GC yield measurement, and they’re covered well.
3) Working Air-Free in the Tolman Lab (PDF, pg 9-12, 17-19)
Similar in form to the Bartlett Schlenk Line guide. This lab uses a “dry box” instead of the standard glovebox, as well as vacuum ovens. The safe use of pyrophoric materials and peroxide genearating solvents is explained in some detail.
Most strong non-nucleophilic bases can be purchased directly from chemical suppliers, but there’s something to be said for the home brew. The Collum lab’s website has detailed instructions for generation of nBuLi, LDA and LiHMDS, with the latter two generated from either nBuLi or isoprene. There’s a wealth of ancillary information as well, from a guide on quenching solvent stills to physical data on a hundred or so enolates, phenolates, carboxylates and miscellaneous alkoxides. Well worth a look.
[1] Incidentally, this is one of the best reasons for hiring post-docs. Because of their prior training a postdoc is likely to have specialties distinct from the rest of the lab, expanding the total toolkit.
[2] See: “How to get that one troublesome HPLC bought in 1993 to work” and other seminal guides.
Filed under: How it's Done, On the Web
Hark, a Vagrant: Alexanders




buy this print!
I had time to make a comic, so I did! Yay COMICS, I miss 'em.
While other stuff is plodding along, and that's great, I wish I could find a way to keep this comic going regularly. Usually, the longer between updates, the more I freak out in my head about how long it's been, and feel like whatever I post next has to be really long, or "worth the wait." But I should just post. Unfortunately, this being the thing that has neither deadline nor paycheque attached, it is always the one that gets pushed back. But I have to figure something out. I really do.
Thanks, everybody, for sending me your Dark Science cast...






Thanks, everybody, for sending me your Dark Science cast suggestions! It was good fun, and there were some great ideas. Here are my favorites:
- Kim Ross - Min Hyo Rin (Sunny). This is an inherently tough cast, so most folks went with a visual similarity. Though I don’t know if she’s fluent in english, Min Hyo Rin’s got chops when it comes to dour/scrappy characters.
- Vonnie Awning - Amber Heard (Zombieland, Drive Angry). Heard’s great at simultaneously glamorous and deranged characters, I think she’s a great choice for Vonnie.
- Balthazar Bogan - Aidan Turner (Being Human, The Hobbit). Turner’s very good at being tossed around and not taken seriously, a necessary skill for Balthazar.
- Kaito Kusanagi - Tadanobu Asano (Ichi the Killer, Thor). I can’t think of a better actor to play the progressively unhinged Kusanagi.
- Alisa Caspar - Vera Farminga (The Departed, Bates Motel). Farminga’s downright brilliant in everything she does. I think she can pull off the casual flair of Caspar.
- Mathias Melchior - Matt Smith (Doctor Who). Matt Smith is very good at shifting from lighthearted to terrifying. He also has an amazing frown.
- D.H. Ron - Benedict Cumberbatch (Sherlock, Star Trek Into Darkness). Think about it, he’s perfect.
Philip
In 1972 the Toronto Society for Psychical Research set out to create a ghost. They invented a character named Philip, an English nobleman from the 17th century, and tried to contact him through sittings in which they discussed his life, to see whether they could induce a “collective hallucination.”
When a year of this produced no results, they adopted the trappings of a more formal séance, introducing colored lights, singing songs, and reciting poetry while trying to conjure Philip’s spirit. After three or four of these sessions, surprisingly, “the group felt a vibration within the table top, somewhat like a knock or rap.”
Philip had, apparently, shown up. After some initial confusion, the group established a convention by which he could express himself — one rap meant yes, two meant no. And he was quite willing to talk:
‘Did you have your own regiment?’ Sid asked.
(Rap) ‘Yes.’
‘Were you wounded in the fighting?’
(Rap, rap) ‘No.’
‘I wonder which battles he fought in,’ Lorne asked. ‘Philip, did you fight at Naseby?’
(Rap, rap) ‘No.’
‘Did you fight at Marston Moor?’
(Rap) ‘Yes.’
‘I wonder what weapons they used?’ Al asked. ‘Did you use pikemen?’
(Rap) ‘Yes.’
‘Would they have had guns of any kind then?’ someone asked.
‘They would have had muskets,’ Lorne said.
(Immediate confirmatory rap) ‘Yes.’
That’s from Conjuring Up Philip, a 1976 account by Iris Owen, a member of the group. With time the rappings grew stronger, and the table would occasionally move around the room and even levitate. Owen wrote, “In addition to sliding across the floor (which incidentally was covered with a thick pile carpet), the table would tilt in various ways, lifting one, two, or three legs, and pivot, sometimes almost dancing.”
It’s hard to know what to make of this. On the one hand, most of Philip’s verbal communications were simply those that the group expected to receive. For example, he said that Charles I had loved cats but not horses or dogs; this isn’t historically accurate, but the questioner was a cat lover. Owen called Philip “a composite of [the group's] own invented imaginations … a character born of their own desire to bring him as much to actuality as they could.”
But the physical tricks are harder to understand. The simplest explanation is that some in the group were deliberately creating the “supernatural” effects, a possibility that all strongly denied. Or perhaps the group really had stumbled into some sort of collective, or wishful, hallucination. Whatever the case, the whole episode shows that even supernatural explorations that are known to be groundless can produce convincingly “otherworldly” effects for a receptive audience. To that extent, Philip was a ghost who helped to disprove his own existence.
Chemical-Free Fruit Washing
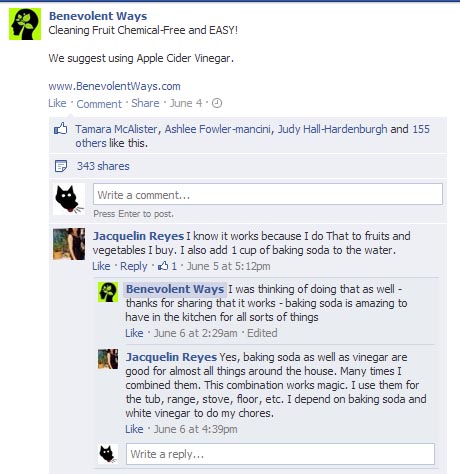
A friend sent me the following picture on Facebook:
Cleaning Fruit – Chemical-free and EASY!
Fill sink with water, add 1 Cup of Vinegar, and Stir. Add all fruit, and Soak for 10 minutes. Water will be dirty, and fruit will sparkle with no wax, or dirty film. Great for Berries too, as it keeps them from molding. Do this with strawberries, and they last for weeks!
SHARE this post with your friends!
Oh, I will share this post with my friends all right…
No chemicals, you say? Fantastic! I am glad you’ve managed to remove the acetic acid impurity from your vinegar. ![]()
Of course, you’ve seen this on ChemBark already. What I find more interesting are the comments attached to the post:
Oh, you think vinegar works well? Have you tried mixing vinegar and baking soda? It’s magic!
What a discovery! “Magic” = sodium acetate + bubbles of carbon dioxide.
I bet these people would be frightened to learn how baking soda is made. It’s not exactly the most “natural” of processes.
H/T: M.E. for the link. Thanks!
Conforiguration
If you show an organic chemist the structures of two stereoisomers, she’ll probably have no issue characterizing them as conformational or configurational isomers. Structures differing in the arrangement of atoms about one tetrahedral stereocenter are naturally configurational isomers; structures differing only with respect to rotation about a single bond are naturally conformational isomers. Baby stuff.
The criterion for making these judgments appears to be whether the isomers are related by one or more rotations about formally single bonds (conformational) or any other sort of topological change (configurational). The terms “configuration” and “conformation” only have meaning, then, when another isomer is considered. Put another way, a single structure can be both a configurational and conformational isomer simultaneously depending on context. That’s not so bad, although the situation causes issues for the novice, to whom the relevant “virtual isomer” may not immediately spring to mind.
The real issue comes to the fore when one considers why the hell “rotation about formally single bonds” is the criterion for conformational isomerism. This is one of those theoretical facts that you’ll tell a student, and afterwards they might just stare back at you blankly (if they aren’t of the disposition to accept theoretical facts at face value). They’ll wonder what’s so special about single-bond rotations.
Upon reflection, you’ll realize that there’s nothing special about bond rotations at all, except that they’re easy at room temperature. Falling further down the rabbit hole, you may conclude that any structures related by an easy process at room temperature ought to be called conformational isomers. This includes, for example, amines containing stereogenic nitrogens related by amine inversion (which occurs with lightning speed at room temperature).
The new definition admits more isomers as conformers and removes the illusory “specialness” of single-bond rotations, but it creates additional problems! All of a sudden, the isomeric relationship between two structures depends on how much energy is available in the surroundings: if the activation energy of interconversion of the isomers is less than the ambient energy (proportional to the ambient temperature), the isomers are conformers; if not, they are configurational isomers. It seems we can transform conformers into configurational isomers simply by lowering the ambient temperature! That doesn’t seem right—whether two isomers are configurational or conformational ought to depend only on their structures, right? What activation barrier corresponds to an “easy” process? Would an easy process on Venus be the same as an easy process here on Earth? Yikes…
The only way to sharply divide conformation and configuration under this new definition is to establish a threshold for activation energies of interconversion, above which isomers are considered configurational. I’ve heard that IUPAC has defined the threshold as 19 kcal/mol (80 kJ/mol). This energy corresponds to a rate of about once per second at room temperature, assuming a frequency factor of 1014 per second. IUPAC’s threshold seems sensible at room temperature, but what about –78 °C or refluxing toluene? To the chemist working at these temperatures, the definition still seems arbitrary.
There really are no general solutions to the ambiguities associated with conformation and configuration aside from setting an arbitrary cutoff point or using the “single-bond rotations only” definition. However, the concept of residual stereoisomerism works well for practical purposes and allows one to throw conformation and configuration out the window. Residual stereoisomers are all the isolable species present in a particular sample under a given set of conditions. 1,2-Dichloroethane consists of one residual stereoisomer at room temperature, but cool the stuff down to 50 K and three (the two enantiomeric gauche structures and one anti structure) emerge. The single isomer at room temperature is best conceived as a “smeared” average of the conformers, weighted by their Boltzmann populations, but it’s tough to argue that the conformers have independent existence at room temperature. Cooling them down makes them isolable from one another, at which point it makes more sense to think about them independently. Whether we call them conformers or configurational isomers at 50 K is irrelevant.

1,2-DCE consists of one “smeared” residual stereoisomer at 298 K, but three residual stereoisomers at 50 K.
Will conformation and configuration ever be purged from the organic chemistry literature? Probably not, as these terms are central to the discipline (despite their ambiguities). Still, residual stereoisomerism is a valuable concept for the practicing chemist, to whom the isolability of a structure is often most important. Eliel aptly calls the condition for the existence of a residual stereoisomer the isolability criterion. Conformers by the second definition fall on the “not isolable” side of the criterion, whereas configurational isomers fall on the “isolable” side.
As an aside, the “single-bond rotations only” definition of conformation does have some value from a topological standpoint, when the student first encounters the idea that groups can rotate about single bonds. In practice however, conformation connotes flexibility and configuration connotes rigidity, and these gut feelings grow stronger with additional studies. It’s worth re-evaluating the rigor of one’s instincts from time to time!
More Fun with Google NGram
Once more, I found myself falling down the NGram rabbit hole...
Derek Lowe's played with this a bit over at Pipeline. One thing his commenters wanted to highlight? Carbene. Turns out, when the first neutral lone pair appeared on carbon (around 1910), not many people wrote it down as such. When v.Eggers-Doering and Simmons / Smith began incorporating carbenes into organic synthesis in the mid-'50s, BOOM! They exploded onto the scene:
Vitamins, which were virtually unknown as food additives impacting human heath in the 1800s, were rapidly identified by the early 1900s. In just 20 short years, they went from nonexistent to appearing three out of a hundred thousand published words:
When van Leeuwenhoek first peered through his first microscopes, he called the protozoa, bacteria, amoebae, and various other biology "animalcules." This persisted for a few centuries, until Pasteur and Koch began to advance the germ theory. The result? In just a few short years, microorganisms - both literal and literary - took over:
Readers - Play around, and tell me if you can find some more fun trends!
I'll update accordingly...
Update (6/8/13) - Thanks Andrew, 0.001% is NOT 1 our of a thousand (D'oh!)
Lab Entrance Exam
 All of a sudden, I’ve started receiving a deluge of e-mail from undergraduate students looking to start research. Given the space limitations, one of my labmates joked that to be fair, I should sponsor a Hunger Games style competition for admission to the lab.
All of a sudden, I’ve started receiving a deluge of e-mail from undergraduate students looking to start research. Given the space limitations, one of my labmates joked that to be fair, I should sponsor a Hunger Games style competition for admission to the lab.
I have never watched or read “The Hunger Games,” but I am somewhat uncomfortable sponsoring any sort of fight to the death. Nonetheless, it is interesting to think of what characteristics you’d like to have in prospective students. Perhaps a proper entrance exam would be more appropriate than mortal combat?
Lab Entrance Exam for Undergraduate Students
The lab entrance exam is held on the first Saturday of each month beginning at 6 am. It is broken down into the following subjects. You may use a calculator. Good luck.
Glassblowing: Given a BBQ grill and a 55-gallon drum of sand, construct a vacuum manifold for your fume hood.
Art of Negotiation: Given a p-card loaded with $50, purchase a stirring hot plate, lab jack, variac, and set of heating mantles for your bench. Hot plate must be IKA-brand or better.
Hazardous Waste Disposal: Using qualitative tests from gen chem and orgo lab, determine whether the unlabeled bottle of frothy orange liquid left in our future lab space should be disposed of as aqueous acidic, aqueous basic, halogenated organic, non-halogenated organic, or heavy metals waste. Fill out an appropriate waste label and carefully move the bottle to the waste collection cabinet.
Visual Acuity: From a distance of two meters, determine absorption maxima for the three solutions of chromophores sitting on the bench. Each chromophore has only one peak in the visible spectrum. Bonus: estimate extinction coefficients.
Safety: Within 15 seconds, locate and activate the lab’s eye wash station after donning a blindfold and being spun around twenty times.
Language/Writing: Translate any article in the journal Tetrahedron into English.
Olfactory Acuity: Given a hard copy of our group inventory, open the stockroom fridge for two seconds and report what bottles are leaking. If you haven’t already fallen unconscious, locate them and tighten the lids.
Skepticism: Write a 2000-word letter detailing the experimental deficiencies of any recent paper by an associate editor or advisory board member of Science, Nature, JACS, or Angewandte. If the editor refuses to publish your letter, upload it to Reddit/chemistry.
Endurance: Collect 1000 10-mL fractions of a streaky porphyrin mixture by flash chromatography. Rotavap each to dryness and collect an NMR spectrum for every 20th fraction.
Shuttle Run: Transport eight 20 L drums of methylene chloride from the VWR stockroom to our lab.
Strength: Steal a belt driven vacuum pump from the lab two floors above ours. Do not use our cart—I don’t want it scratched or stained with oil.
Instrumentation: Using a pair of yellow dishwashing gloves, duct tape, a cylinder of carbon dioxide, and anything you can buy at The Container Store for under $100, construct a glove-box suitable for our work simulating the prebiotic atmosphere.
Manual Dexterity: Replace the regulator on a nitrogen cylinder using no tools but your bare hands. Fastest time wins maximum points, but leaks in the system will result in penalties.
_
I’d be happy to take any students who could finish these tasks, even if they were lazy and skipped one or two of them.
PowerPoint Peeves and Chemistry Arrows
ChemBark’s ongoing series on PowerPoint advice continues with a look at another one of my irrational pet peeves: the “fat” reaction arrow.
If you are writing reactions in a text box, chances are you’ll use the “rightwards arrow”, Unicode character 2192 (hex). You will probably also choose a font without serifs, because slide text (including atomic symbols) looks better without serifs. Unfortunately, this is how the rightwards arrow is rendered in many sans-serifs fonts:
I know this is a matter of personal taste, but there is something about that reaction arrow that turns me off. It’s too fat. The same character in Times New Roman looks much better. She has a graceful elegance relative to her Calibri cousin. Consequently, if you want to stick to Unicode characters for writing out reactions, I advocate writing everything in a sans-serif font, then changing the arrow to Times New Roman. You many also need to adjust its font size to make it look right:
And while we’re on the subject of arrows, there is no need to use the double-headed arrow symbol U+2194 (↔) to indicate an equilibrium. Remember, the double-headed arrow typically separates resonance structures. Instead, use U+21c4 (⇄) or U+21cc (⇌). These characters don’t always appear on the maps in Office, but you can copy and paste the symbols into your text. A full listing of arrow characters is here.
Lab Manuals
 I’m always interested to come across instructional documents on chemistry professors’ Web sites. These documents can be great resources, because they often contain very practical advice about safety, direction on how to maintain instruments, and guidance on experimental technique from experts in the field. Taking the time to commit this information to writing also helps prevent “institutional” loss of memory when senior members of the lab graduate without having properly trained the next generation of students.
I’m always interested to come across instructional documents on chemistry professors’ Web sites. These documents can be great resources, because they often contain very practical advice about safety, direction on how to maintain instruments, and guidance on experimental technique from experts in the field. Taking the time to commit this information to writing also helps prevent “institutional” loss of memory when senior members of the lab graduate without having properly trained the next generation of students.
Unfortunately, you don’t come across that many lab manuals online. Perhaps this is because some of them are distributed in hard copy only. Perhaps, some professors don’t want to explicitly write procedures and safety guidelines in fear they might be used against them in court. My guess, however, is that most people can’t find the time to sit down and write out this information—or they don’t see the value in doing so.
Jim Tour’s “Guidelines for Research” is among my favorite documents. He gets very specific about some of the advice he doles out. For instance, all nitrogen bubblers left on overnight should have a flow rate of one bubble per second or less. Tour provides guidance on how he likes notebooks to be kept, and he also provides expectations about work ethic and vacations. Finally, there is the passage on personal hygiene:
Personal Hygiene: Although not customary in all countries, Americans generally bathe at least several times per week. As a result, many Americans are offended by the infrequent bathing habits of others (whether Americans or internationals). Thus, you may be leaving a negative impression of yourself without ever knowing it. Unfortunately, bad impressions are often difficult to overcome. Likewise, be sure to use an underarm deodorant since most Americans find body odor to be most offensive. I have seen people causing themselves to be ostracized by others simply because of poor personal hygiene habits.
It might seem trifling or overbearing to provide advice on this level, but the info is correct and I wish more people heeded Tour’s advice.
While the idea of writing a manual all at once seems daunting, I think that doing it in pieces seems quite reasonable. In fact, I think you can assemble some really good tidbits of advice from material that is already posted online. These documents are almost like official memoranda to members of professors’ labs. For instance:
The famous “How to Write a Scientific Paper” article in Advanced Materials had its beginnings as a type-written memo from George Whitesides to his lab.
There’s also Ken Suslick’s cool presentation on how to give a talk.
And I like how some professors provide specific instructions on how to ask them for letters of recommendation.
Anyway, before I go writing similar stuff in the future, I wanted to know if you all had come across any great lab manuals or memos. Leave them in the comments, and I’ll compile a list below.
Lab Manuals
Jim Tour’s “Guidelines for Research”
Melanie Sanford’s “Group Welcome Kit”
Dave Collum’s site
Bart Bartlett’s “Standard Operating Procedures”
Turro Group’s site
Watson Group Manual
Tolman Lab’s “Standard Operating Procedures”
Armen Zakarian’s site
Rearrangement of 4-Amino-3-halo-pyridines by Nucleophilic Aromatic Substitution
Enantioselective Synthesis of (Thiolan-2-yl)diphenylmethanol and Its Application in Asymmetric, Catalytic Sulfur Ylide-Mediated Epoxidation
Speak Up!
 |
| Louder, Yorick, I can't hear you! Source: RSC |
I can never hear anyone's questions!
We scientists aren't always the most talkative types, but you'd think we could eke out some volume when it counts, right?
Wrong. I can't tell you how often I've cupped my hand to my ear, leaned so far forward I'm nearly doubled over, and all I get are vague "Charlie Brown's teacher" noises directed at the seminar speaker.
Thoughts race through my head: "Was that my question? Will the speaker repeat it? Don't we have a microphone around???"
Well, let's try to set some ground rules to follow before the next occurrence.
Physiology - Ever take voice or speech classes? There's some easy steps to take to project your voice:
1. Prepare. Don't fumble for words or go 'round in logical circles. One of my colleagues writes his question down on paper before he asks, and then reads from the script. Try it.
2. Breathe. Before you speak, take a deep breath, filling bottom-to-top (diaphragm to chest). Your air supply governs your voice, so fill up!
3. Open up. Your soft palate, the tissue near the back and top of your mouth, needs to be open to allow your voice to resonate in your nasal cavities. One trick to accessing this space? Pretend to yawn, but stop yourself before you do. Feel that heightened, awake moment? That's the soft palate moving, permitting increased air flow.
4. Speak. Use full sentences, and make sure you're communicating the central point of your question. The goal is for the speaker - and the audience - to hear and understand what you're asking. Pause as necessary, using measured spaces between words to drive home important points.
Etiquette - Never just shout out your question, or attempt to cut someone else off while asking theirs.
It's not necessary to overly praise the speaker for his unbelievable oh-my-gosh best talk I've ever heard in my life so thoughtful and well-arranged, etc, etc. The speaker knows they're competent, or they wouldn't be at the front of the room, lecturing...
It's also unnecessary to show your wittiness and intellect by recounting your personal lab highlights, or how much literature you've read on the topic. It's the speaker's moment, not yours.
Always use common courtesy: "Please," "Thank You," "You're Welcome," "Professor," "Dr," "Sir," "Miss / Madame," "Excuse Me," "May I." A few gracious words in the right circumstance could catch the eye of a future collaborator or postdoc advisor.
All else fails? Ask the speaker after the talk, when you can get them one-on-one.
Readers: Have more tips for our seminar questioners? Talk to me in the comments!